N1 N2 N3 N4 Chemistry

Quantum Numbers
Q Tbn 3aand9gcryadcko5qh56frjjrqxhnao2jw1gwoufhwij 0qwsubfwr7fyj Usqp Cau

Syntheses Structures And Spectroscopic Properties Of 1 10 Phenanthroline Based Macrocycles Threaded By Ptc8pt Ptc12pt And Ptc16pt Axles Metal Capped Rotaxanes As Insulated Molecular Wires Amini 19 Chemistry 11 A European Journal
6 3 Line Spectra And The Bohr Model Chemistry Libretexts

The Crystal Structure Of Tris Thiourea Copper I Perchlorate Cu Scn2h4 3clo4

The Change Plot Of The Concentration Of Chemical Pollutants In The Download Scientific Diagram
Could sombody show me how to do it so i can know what im doing wrong?.
N1 n2 n3 n4 chemistry. 1 n = 1;. (1)College of Chemistry, Fuzhou University, Fuzhou , People's Republic of China. Get hold of all the important DSA concepts with the DSA Self Paced Course at a student-friendly price and become industry ready.
Assume that the equation is true for n, and prove that the equation is true for n + 1. For real-valued orbitals, as convenient in chemistry, look at Hydrogen orbitals 3D real. For integer n ≥ 1.
Balmer Series (to n=2) n=3 to n=2:. Simplifying 24 + 50n + 35n 2 + 10n 3 + n 4 = 0 Solving 24 + 50n + 35n 2 + 10n 3 + n 4 = 0 The solution to this equation could not be determined. How many orbitals in an atom can have the designation:.
It has no other electrons. Which electronic transition in atomic hydrogen corresponds to the emission of visible light?a) n = 5 → n = 2b) n = 1 → n = 2c) n = 3 → n = 4d) n = 3 → n = 1. It is possible to determine the ionization energy for hydrogen using the Bohr equation.
4, 16, 64, 256) 1 n is going to be 1 always, independent of n. As documented by Peter Borwein, prime factorization allows n!. When i did the first one i got 1.60*10^14, which was right, but when i did the second one the same way i got it wrong.
N = 1. Since contain both numbers and variables, there are four steps to find the LCM. + n = (n(n+1))/2 for n, n is a natural number Step 1:.
Lyman Series ( to n=1) n=2 to n=1:. To be computed in time O(n(log n log log n) 2), provided that a fast multiplication algorithm is used (for example,. I mean, look at it for a second.
2 1 (— • (n + 1)) - (0 - (— • n)) = 0 3 2 Step 2 :. It converts toxic ammonium ion to urea which is then excreted from the body in urine. Solve for n 2(n-3)=4n+1.
Of the following transitions in the Bohr hydrogen atom, the ____ transition results in the emission of the highest-energy photon. Join Yahoo Answers and get 100 points today. A neutral atom has two electrons with n = 1, eight electrons with n = 2, eight electrons with n = 3, and one electron with n = 4.
3 n = 3;. How much energy would an electron have to gain to move from n = 1 to n = 4?. We have step-by-step solutions for your textbooks written by Bartleby experts!.
We know that 1^3+2^3+3^+n^3 = (1/4){n(n+1)}^2. 1 + 2 + 3 +. 6 n = 6;.
What is the atomic number of this element?. 4 n = 4;. N=7 → N=5 N=6 → N=5 N=4 → N=7 N=1 → N=4 N=3 → N=5 N=1 → N=2 N=3 → N=1 N=2 → N=1.
What is(are) the solution(s)?. Are all part of an empirical theory designed to explain what we observe with respect to molecular structure and bonding. All atomic orbitals with n=10 are presented here.Note that the orbitals with negative m are identical to those with the same magnitude positive m value except for a rotation,and are not shown separately.
As the energy of the electron increases, so does the principal quantum number, e.g., n = 3 indicates the third principal shell, n = 4 indicates the fourth principal shell, and so on. Search results for N-(2-3,4-dihydroxyphenylethyl)acrylamide at Sigma-Aldrich. N=4 -> n=3 n=5 -> n=1 n=5 -> n=4 n=6 -> n=5.
Finding the LCD of a list of values is the same as finding the LCM of the denominators of those values. Example 12 Find the value of n such that nP5 = 42 nP3, n > 4 Given nP5 = 42 nP3 Calculating nP5 nP5 = 𝑛!/(𝑛 − 5)!. The title coordination polymer, {Cd2(CH2N5)(C6H4NO2)Cl(OH)·0.14H2O}n, (I), was synthesized by the reaction of cadmium acetate and N-(1H-tetrazol-5-yl)isonicotinamide in aqueous ammonia, using hydrochloric acid to adjust the pH.
The orbitals are presented in six different ways, n and l versus m, n and m versus l, l and m versus n, n-l and l-m versus m, n-l and m versus l-m, and l-m and m versus n-l. 5 n = 5;. That means that the total number of compare/swaps you have to do is (n - 1) + (n - 2) +.
$\endgroup$ – half-integer fan Feb 2 '13 at 13:. Thus you don't have to sort the whole thing every time:. Textbook solution for General, Organic, and Biological Chemistry 7th Edition H.
$\begingroup$ Note, if you wanted to subvert the problem stated, you could perform induction separately on $\sum n^2$ and $\sum n$. 1 Simplify — 2 Equation at the end of step 1 :. Our solution is simple, and easy to understand, so don`t hesitate to use it as a solution of your homework.
Stack Exchange network consists of 176 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. 028 1.0 points A neutral atom has two electrons with n = 1, eight electrons with n = 2, eight electrons with n = 3, and one electron with n = 4. 5 • (n 4-n 3 +n 2-n+1) •.
Prove 1 + 2 + 3 +. Move all terms not containing to the right side of the equation. Found change of sign between n= -2.00 and n= -1.00.
If the I excitation potential of a hypothetical H-like atom is 1.62 V, then the value of II excitation energy is. To find the lt (1^3+2^3+n^3 )/n^4 as n--> infinite. Product of the integers 3.
@Henry While I agree about the sum there are n terms here, thus it is O(n), not O(n^2). – Loren Pechtel May 30 '17 at 3:57 @LorenPechtel no, "which I run through doing whatever" implies you do O(n) work for the first term alone. Which of the following transitions for an electron in a hydrogen atom will absorb the largest energy?.
Prove that the equation is valid when n = 1 When n = 1, we have (2(1) - 1) = 1 2 , so the statement holds for n = 1. A.n=1, n=6 b.n=6, n=1 c.n=6,n=3 d.n=3,n=6 e.n=1,n=4. Find an answer to your question n(n+1)(n+2)(n+3)(n+4) Kylie borrowed a book from the library the library charge to fix rental for the book in late fee for every day the book was overdue expression below s ….
Move all terms containing to the left side of the equation. 6.003 Homework #3 Solutions / Fall 11 3 3. Get 1:1 help now from expert Chemistry tutors.
This problem has been solved!. Z transforms DeterminetheZtransform(includingtheregionofconvergence)foreachofthefollowing signals:. Solve for n 1/(n-4)-2/n=3/(4-n) Find the LCD of the terms in the equation.
Na is basically Ne 3 s 1 and Al is Ne 3 s 2 3 p 1. λ 1 − R Z 2 (n 1 2 1 = n 2 2 1 ) Now answer the following question. Tap for more steps.
To do this, we will fit two copies of a triangle of dots together, one red and an upside-down copy in green. This subproblem is being ignored because a solution could not be determined. (Integer division) The number n can be as large as 10^12, so a formula or a solution having the time complexity of O(logn) will work.
The sum of (1+2^n)/3^n = 1/2 +2 =5/2. What is the atomic number of this element?. Simple and best practice solution for 3(n-4)=2(n-1) equation.
Approximating a root using the Bisection Method :. The wavelength of the photon emitted upon an electronic transition from n 2 to n 1 orbit in a H-like species is given by the formula:. 0ne transition between energy states of the hydrogen atom is represented by the picture on the left.
It is important to note here that these orbitals, shells etc. What electron transition in a hydrogen atom, ending in the orbit 4, will produce light of wavelength 2170 nm ?. 1^2 + 2^2 + 3^2 +.
Stephen Stoker Chapter 26 Problem 26.75EP. < (n / 2) n. It has no other electrons.
Last digit of 4 n always repeat for next 2nd value of n.(ex:. N=infinity n=4 n=3 n=2 n=1 In the Bohr model of the hydrogen atom, the electron occupies distinct energy states. Don’t stop learning now.
To characterize ammonium ion in terms of nitrogen content as N 1, N 2, N 3, or N 4. X 1n = 1. Factorial sign(!) is used to denote the product n.(n-1).(n-2).(n-3).(n-4)……….4.3.2.1.
+ n = (n(n+1))/2 Step. So, If we will have a close look for periodicity of f(n) = (1 n +2 n + 3 n + 4 n ) we will get that its periodicity is also 4 and its last digits occurs as :. (the given statement)\ Let P(n):.
2 n = 2;. N=1, l=0, m=0 n=1, l=0, m=0 n=1, l=0, m=0 n = 2. Subtract from both sides of the equation.
If observed closely, we can see that, if we take n common, series turns into an Harmonic Progression. The Bisection Method is an iterative procedure to approximate a root (Root is another name for a solution of an equation). Selecting "AUTO" in the variable box will make the calculator automatically solve for the first variable it sees.
Calculate the wavelength, in nanometers, of the spectral line produced when an electron in a hydrogen atom undergoes the transition from the energy level n = 2 to the level n = 1. 5 p 3 s n = 4 4 d n = 3 There is a shorthand notation that can be used. In mathematics, the harmonic series is the divergent infinite series ∑ = ∞ = + + + + + ⋯.
So S is composite. Using the above formula it is easily shown that for all n we have (n / 3) n < n!, and for all n ≥ 6 we have n!. You only need to sort n - 2 elements the second time through, n - 3 elements the third time, and so on.
This is an arithmetic series, and the equation for the total number of times is (n - 1)*n / 2. Therefore Lt (1^3+2^3+3^3+4^3+.+(n-1)^3+n^3)/n^4 = lt (1/4. + 2^n = 2^(n-1) This makes absolutely no sense.
Apply the distributive property. The graph of a quadratic equation is shown below. Check how easy it is, and learn it for the future.
Its name derives from the concept of overtones, or harmonics in music:. Set the factor '(24 + 50n + 35n 2 + 10n 3 + n 4)' equal to zero and attempt to solve:. Let mathS=n^4+n^2+1/math mathS=n^4+2*n^2+1-n^2=(n^2+1)^2-n^2/math mathS=(n^2+1-n)(n^2+1+n)/math For n>1, S has two factors other than 1.
F(n) = 4 n is periodical for n = 2 in terms of last digit. I wonder if there is a formula to calculate the sum of n/1 + n/2 + n/3 + n/4 +. Calculate the energy of the 4th electron found in the n = 2 state of the boron atom in kilojoules per mole.
Get answers by asking now. Step by step solution :. Firstly you failed to notice the pattern correctly since 2^n means 2^1+2^2+2^3 instead of what is shown.
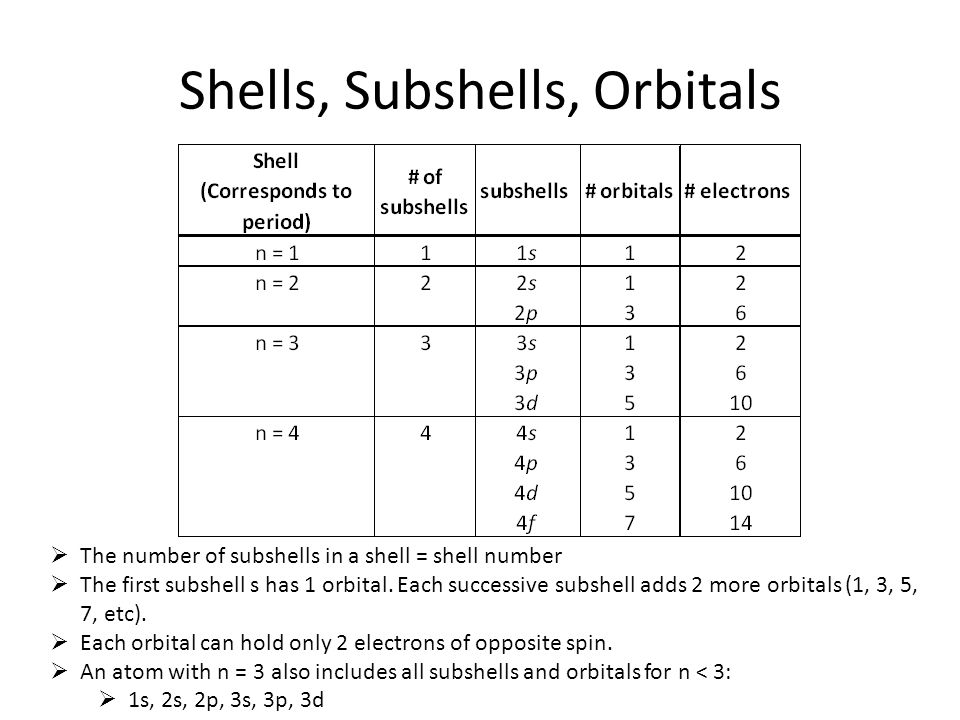
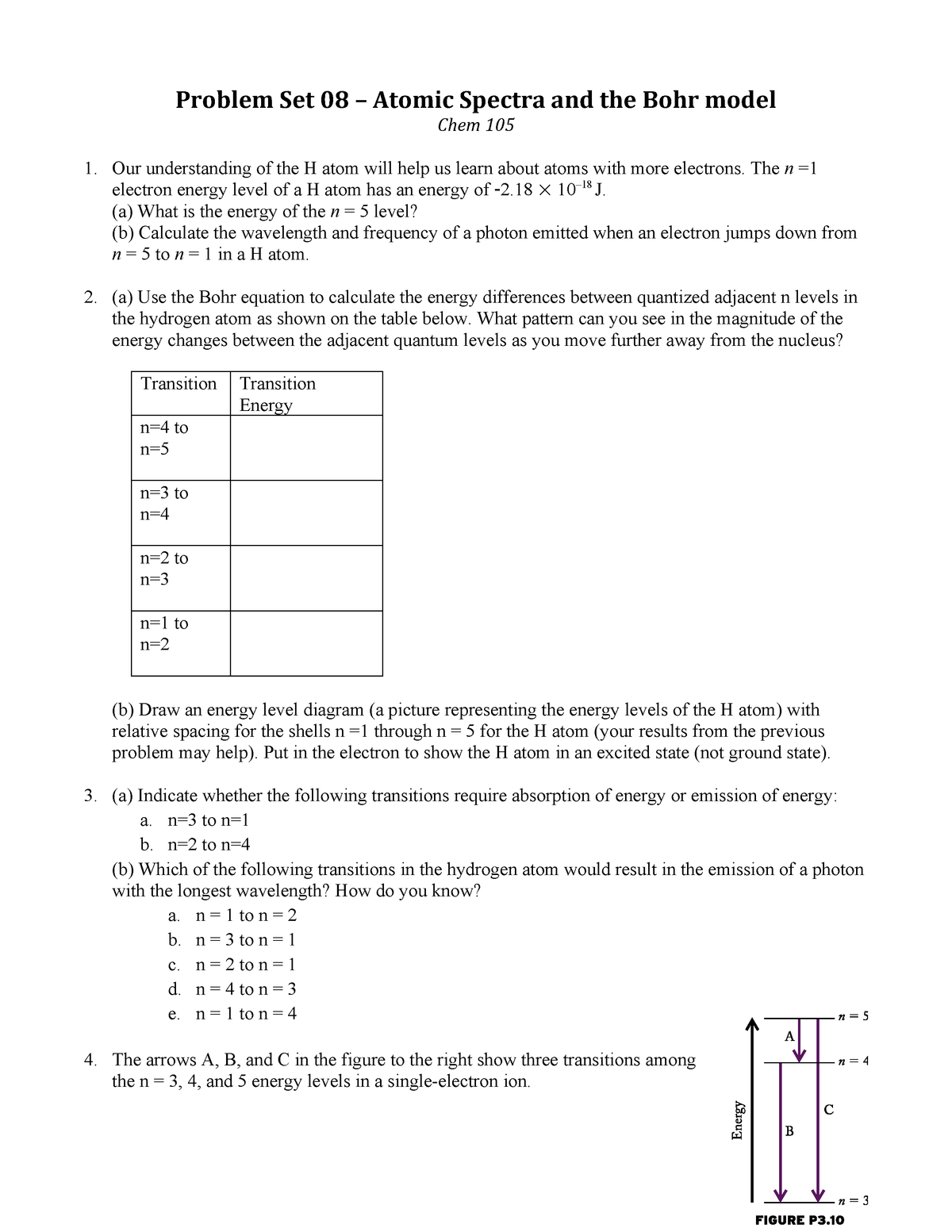
In the n=1 shell you only find s orbitals, in the n=2 shell, you have s and p orbitals, in the n=3 shell, you have s, p and d orbitals and in the n=4 up shells you find all four types of orbitals. N=2 to n=1 n=1 to n=3 (Note:. Tap for more steps.
As you get to higher energy shells, the difference between becomes smaller) When an electron in a hydrogen atom is in the n=3 state, is it on average closer to, farther from, or the same distance to the nucleus than in the ground state?. The wavelengths of the overtones of a vibrating string are 1 / 2, 1 / 3, 1 / 4, etc., of the string's fundamental wavelength.Every term of the series after the first is the harmonic mean of the neighboring terms;. Urea cycle is a cyclic biochemical pathway that involves the production of urea using ammonium ions and aspartate molecules as nitrogen sources.
Tap for more steps. N=2, l=0, m=0 n=2, l=0, m=0 n=2, l=0, m=0 n=2, l=1, m=-1. We now use the Bisection Method to approximate one of the solutions.
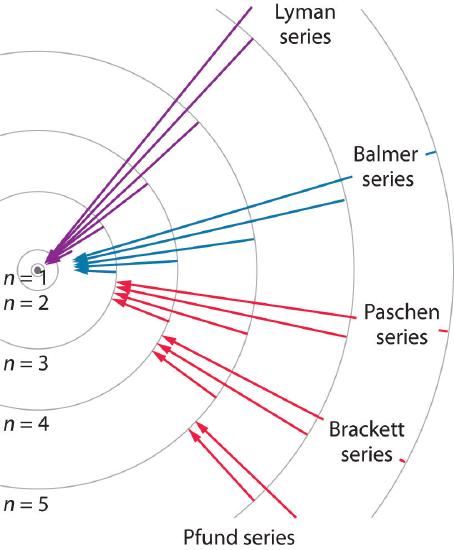
Known as emission, electrons can also "emit" energy as they jump to lower principle shells, where n decreases by whole numbers.

Chemistry For The Ib Diploma Second Edition By Cambridge University Press Education Issuu

Ppt Chemistry A Molecular Approach 1 St Edition Nivaldo J Tro Powerpoint Presentation Id
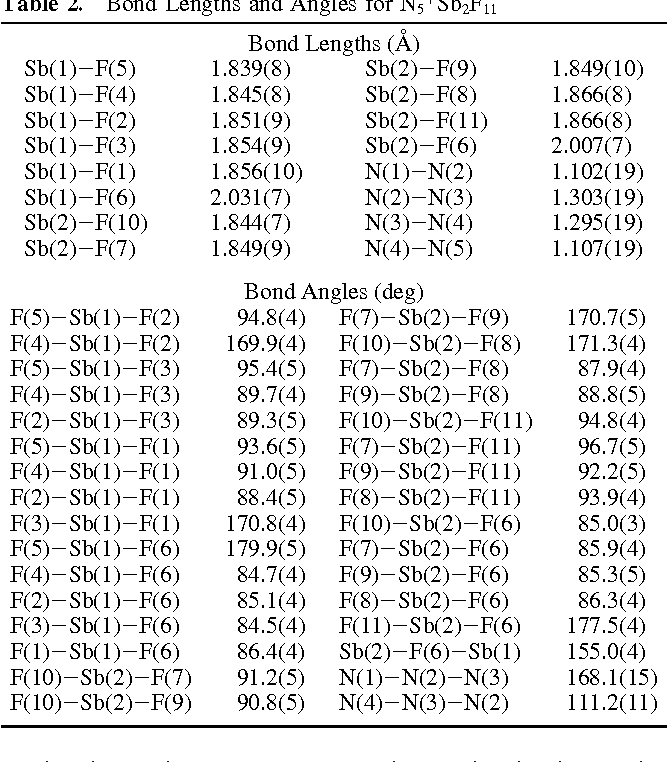
Table 2 From Polynitrogen Chemistry Synthesis Characterization And Crystal Structure Of Surprisingly Stable Fluoroantimonate Salts Of N5 Semantic Scholar
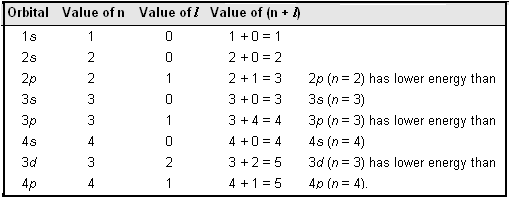
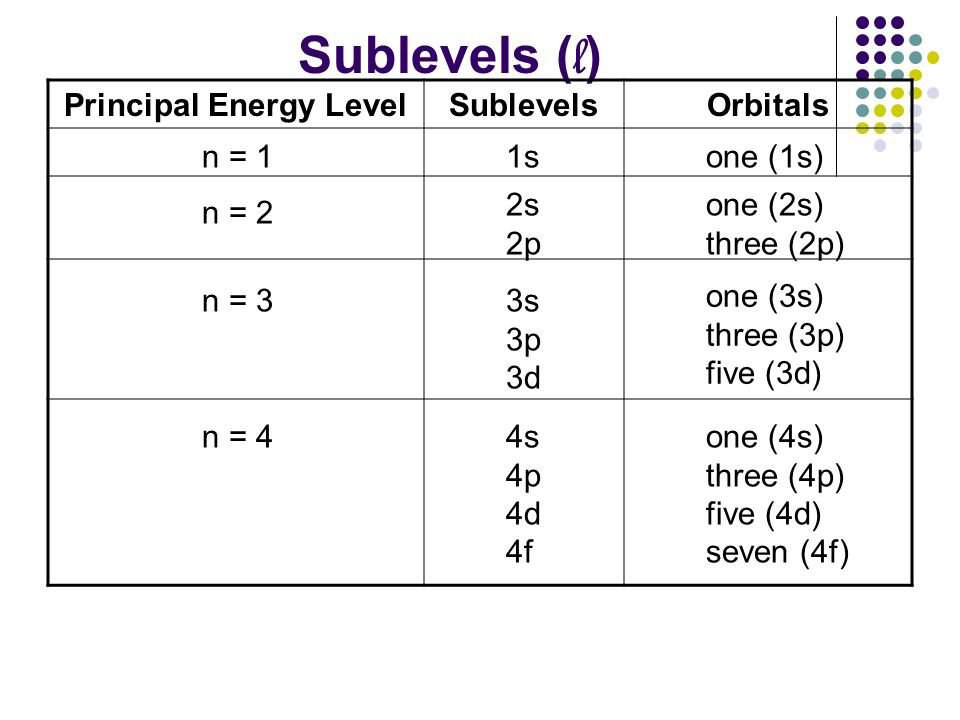
Subshell Shell N L Rule Value Of L May Be 0 1 2 3
What Is The Difference Between A Shell And A Subshell Socratic

Crystal Structure Of N 4 Oxo 2 Sulfanylidene 1 3 Thiazolidin 3 Yl 2 Thiophen 3 Yl Acetamide
Solved I Have Two Chemistry Questions From My Test Review Chegg Com
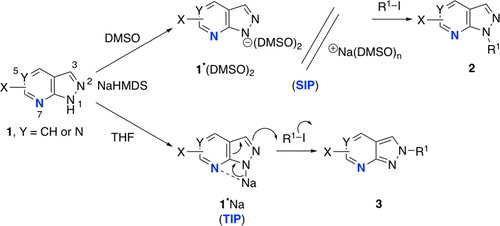
Solvent Controlled Site Selective N Alkylation Reactions Of Azolo Fused Ring Heterocycles At N1 N2 And N3 Positions Including Pyrazolo 3 4 D Pyrimidines Purines 1 2 3 Triazolo 4 5 Pyridines And Related Deaza Compounds J Org Chem X Mol
Chemistry Understand Electronic Configuration Studying Amino Amino

Structure Of The Atom Ncert Solution Chemistry Class Xi Sharya Academy

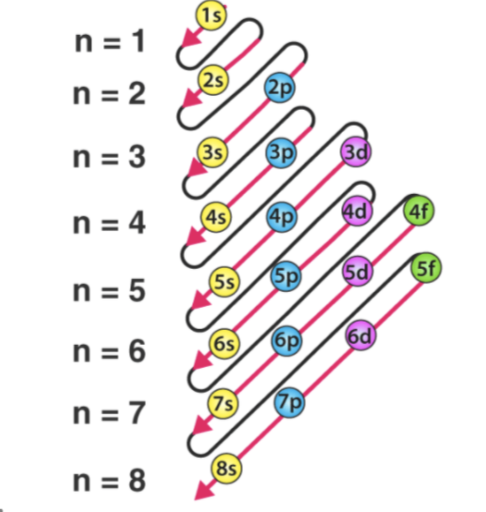
Diagnol Rule

Quantum Numbers And Electron Configurations

7 1 Orbitals Of Polyelectronic Atoms Chemistry Libretexts
Bohr Model Wikipedia

The Quantum Mechanical Model Of The Atom Ppt Video Online Download

Atomic Structure And Properties Sch4u Catherine Gordeyev

The Changes Of The Concentration Of Chemical Pollutants In The Aquifers Download Scientific Diagram
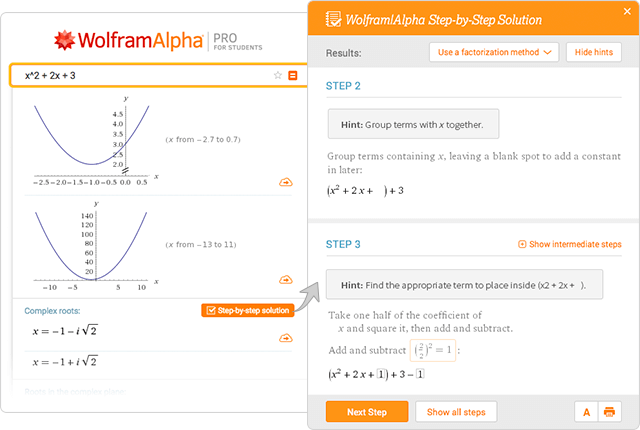
Wolfram Alpha Examples Step By Step Solutions

Determine Whether Each Of The Following Transitions In The H Clutch Prep
N 1 2 Hydroxy 3 Methoxybenzyl 4 Piperidinyl 2 Methoxy N 4 Methylphenyl Acetamide C23h30n2o4 Chemspider
Ps 08 19 Taanswers Chem 105 General College Chemistry Studocu
Chapter 2 5 Atomic Orbitals And Their Energies Chemistry Libretexts

Honors Chemistry Worksheet Electronic Structure Of Atoms

Ap Chemistry

An Electron In A Hydrogen Atom Could Undergo Any Of The Tran Clutch Prep

Don Mencer S Site
Www Myhaikuclass Com Kristakyoung Chm080 Cms File Show Pdf T
Pubs Acs Org Doi Pdf 10 1021 Ed011p51 Rand Jpa90bpv

Chemical Principles 5th Edition Atkins Test Bank

January 19 The Math Citadel
Bohr Model Wikipedia
Chemidplus 610 27 0 Iembuwhxcnuyst Uhfffaoysa N 1 3 5 Triazine 2 4 6 Triamine N2 N2 Bis 3 4 6 Bis Butyl 2 2 6 6 Tetramethyl 4 Piperidinyl Amino 1 3 5 Triazin 2 Yl Amino Propyl N4 N6 Dibutyl N4 N6 Bis 2 2 6 6 Tetramethyl 4 Piperidinyl
File Chemistry From Az

Electronic Structure Of Atoms Chemistry Library Science Khan Academy
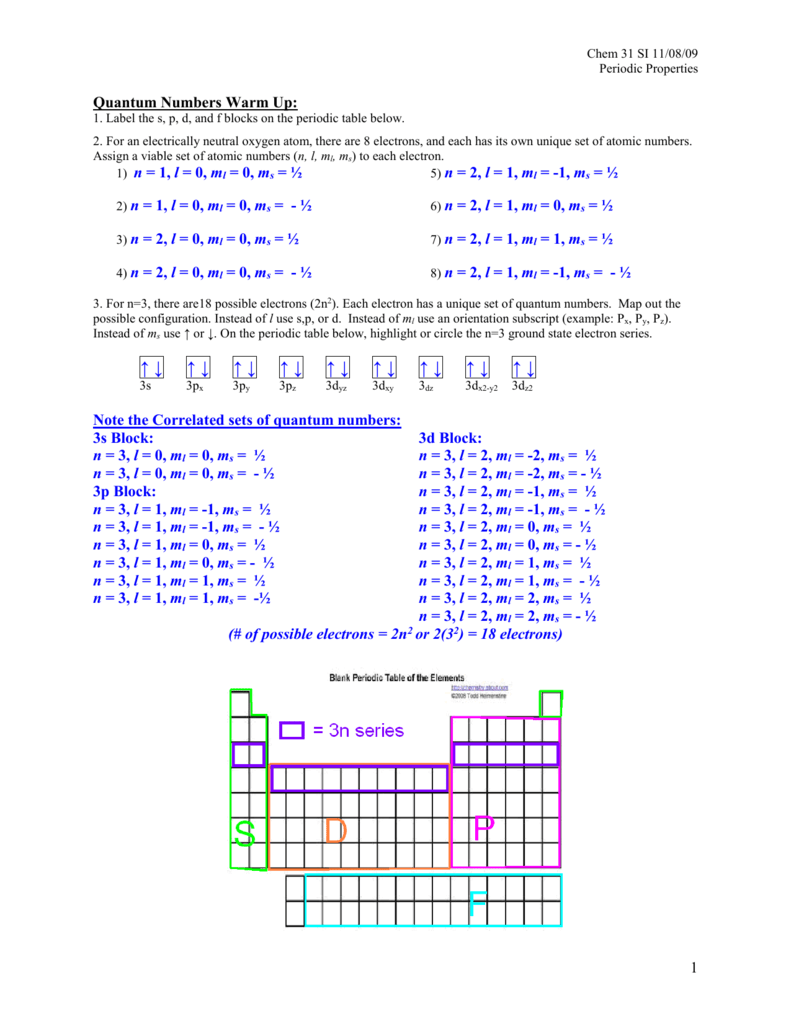
November 8 Periodic Properties Solutions

1 Hse I Part Iii Chemistry Max Marks 60 Max Time 2 Hrs Cool
Solved I Whtch Tune Of The Fullnwing Sets At Quantum Numbers Is Nut Possible Course Hero
Www Morehouse Edu Media Chemistry Brianlawrence Genchem 111problems Ps08 Pdf
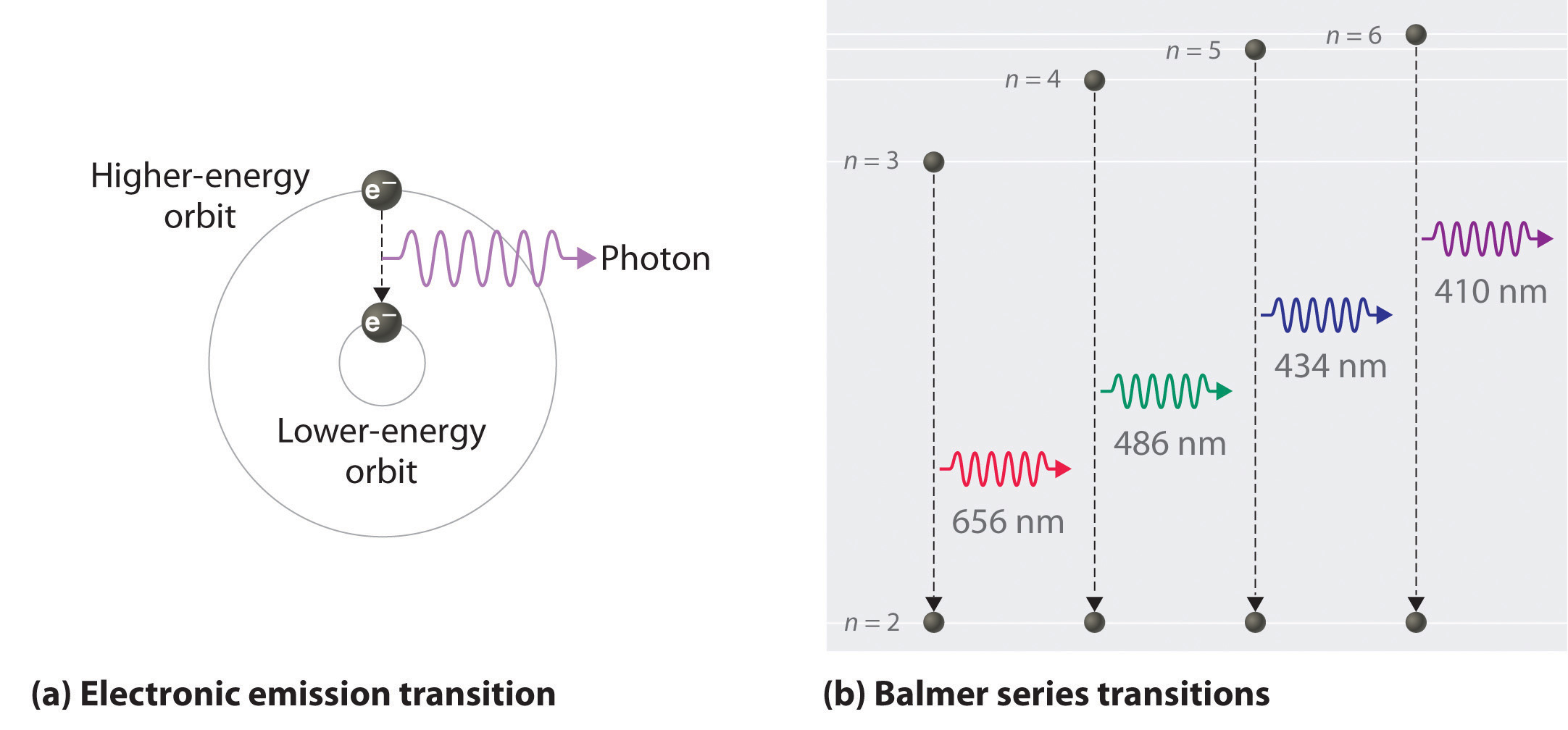
6 3 Line Spectra And The Bohr Model Chemistry Libretexts
1
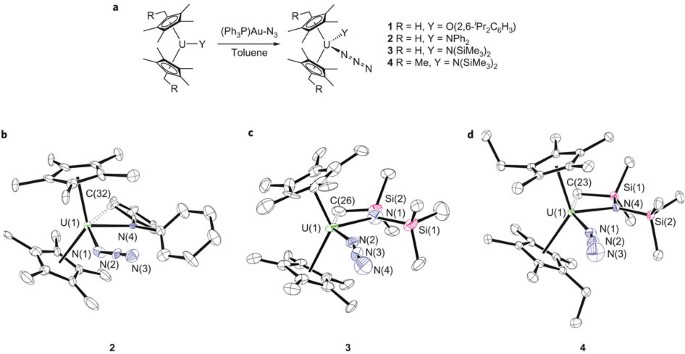
Uranium Azide Photolysis Results In C H Bond Activation And Provides Evidence For A Terminal Uranium Nitride Nature Chemistry
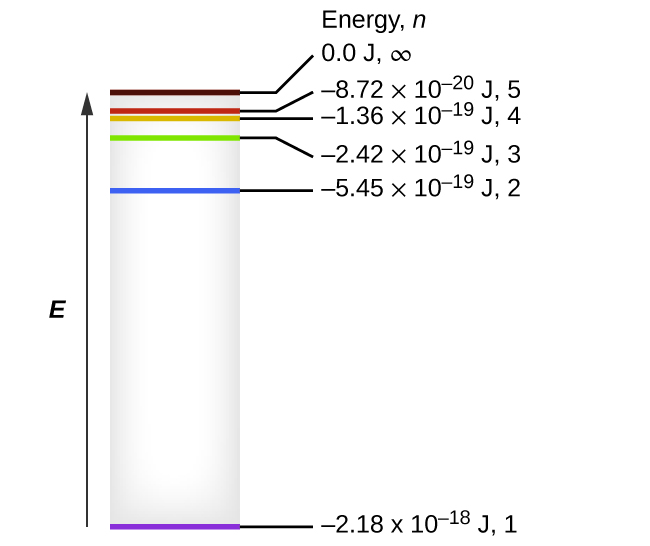
6 2 The Bohr Model Chemistry

Chem 1411 Practice Examsample Practice Exam Chem 1411 Studocu

Click On A Subshell To See The Labels For All Orbitals In Th Clutch Prep

Hexachlorophosphazene Wikipedia

Synthesis Biological And Antitumor Activity Of A Highly Potent 6 Substituted Pyrrolo 2 3 D Pyrimidine Thienoyl Antifolate Inhibitor With Proton Coupled Folate Transporter And Folate Receptor Selectivity Over The Reduced Folate Carrier That Inhibits B
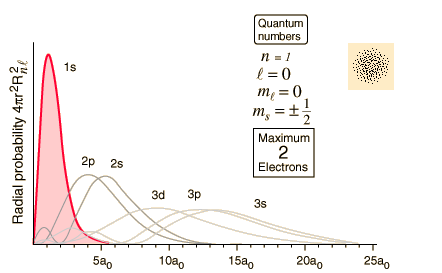
Orbitals

Unit 4 Cp Chemistry Bohr Quantum Numbers Quantum Mechanical Model Ppt Download
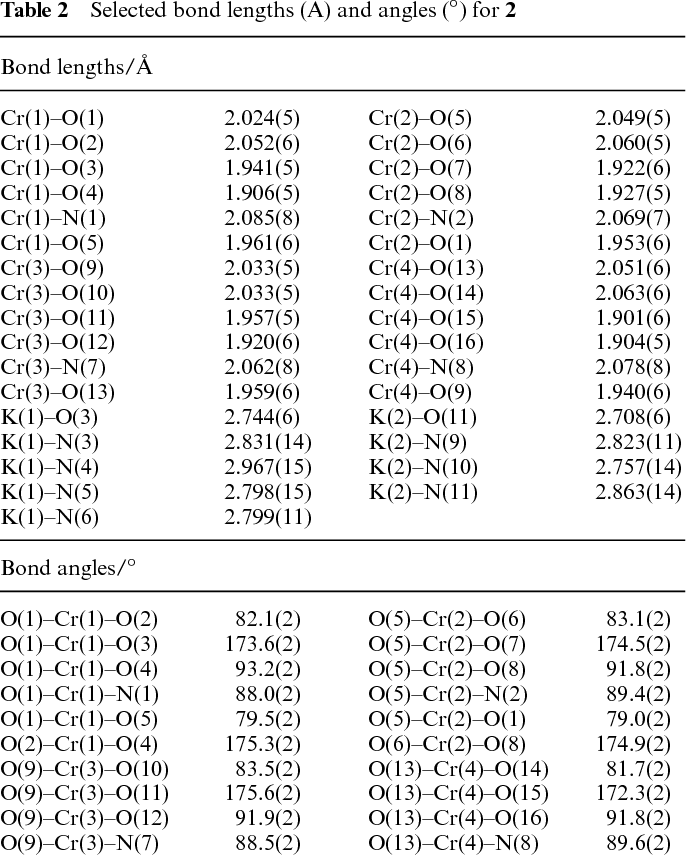
Table 2 From New Structural Motifs In Chromium Iii Calix 4 And 6 Arene Chemistry Semantic Scholar

Neet Chemistry Mcq Atomic Structure 15 Youtube
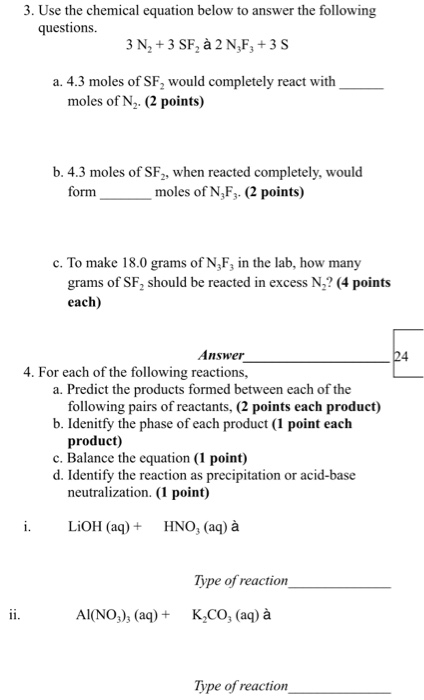
Solved 3 Use The Chemical Equation Below To Answer The F Chegg Com

Chemical Structure Of The N 4 Cyclohexyl 1 2 4 Triazole 3 Thione 2 Download Scientific Diagram
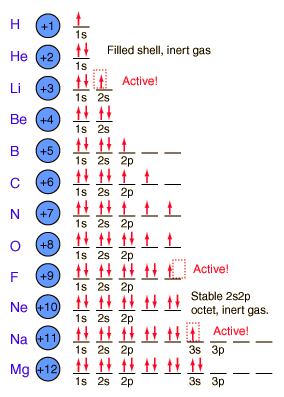
Orbitals

Using S P D D Notation Describe The Orbitals With The Following Quantum Numbers 1 N 2 L 1 2 N 4 L 0 3 N 5 L 0 M 0 4 N 4 L 2 Chemistry Structure Of Atom Meritnation Com

Question And Problem Set With Answers Orbitals Chemistry 105 Studocu

Atomic Sub Shells

Quantum Numbers And Orbitals

Ch 101 Chemistry A Molecular Science At Ncsu Clutch Prep
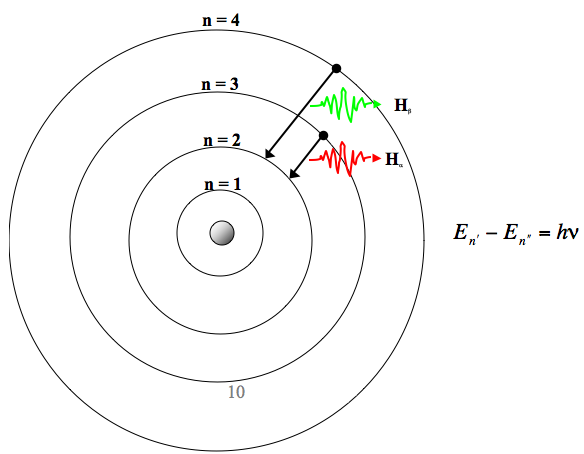
Bohr S Atomic Model Line Spectrum Of H Atom Zeemann Stark Effect

Chem 110 Study Guide Fall 18 Final Bohr Model Electron Configuration Formal Charge
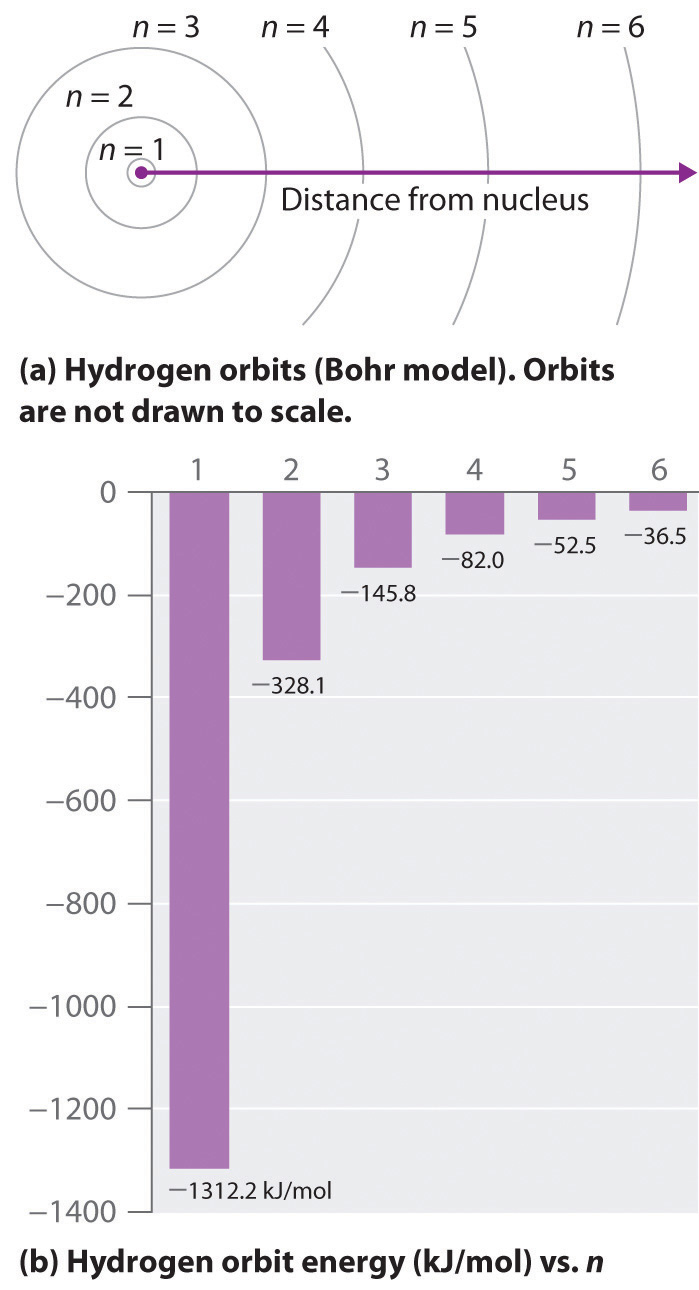
7 3 The Atomic Spectrum Of Hydrogen Chemistry Libretexts

Chem Quiz 7 Diagram Quizlet

1 Electronic Structure Of Atoms Periodic Table Chapter 4 Chemistry Dacs 1232 Fakulti Kejuruteraan Mekanikal Utem Lecturer Imran Syakir Bin Mohamad Ppt Download
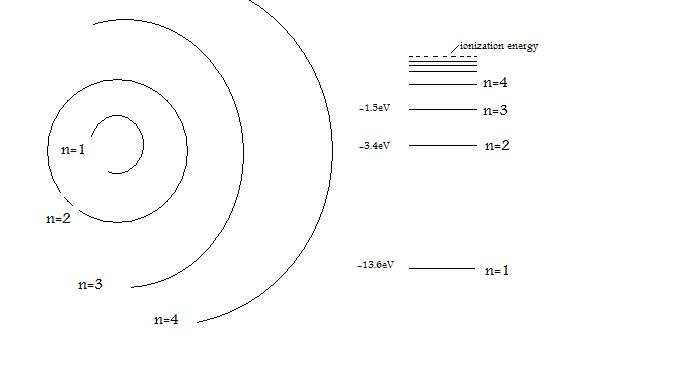
Bohr S Hydrogen Atom Chemistry Libretexts
Mrosechemistry Weebly Com Uploads 2 7 5 8 1a Quantum Numbers Pdf

Chapter 11 Modern Atomic Theory Chemistry 101 Structure Of Atom Rutherford S Model Source Of Particles E E Ppt Download

N W Purin 6 Yl Aminoalkanoyl Derivatives Of Chiral Heterocyclic Amines As Promising Anti Herpesvirus Agents Krasnov 19 European Journal Of Organic Chemistry Wiley Online Library

Objective Test 2 On Quantum Numbers Mm 30 Time 45 Min
1

Chem 1411 Study Guide For Test 3 Chapters 7 8 9 Pages 1 12 Flip Pdf Download Fliphtml5
Q Tbn 3aand9gcqy Adduyo2xtthvrgj 0sqpilzworaodzed6ftay7e3ebj2dl2 Usqp Cau
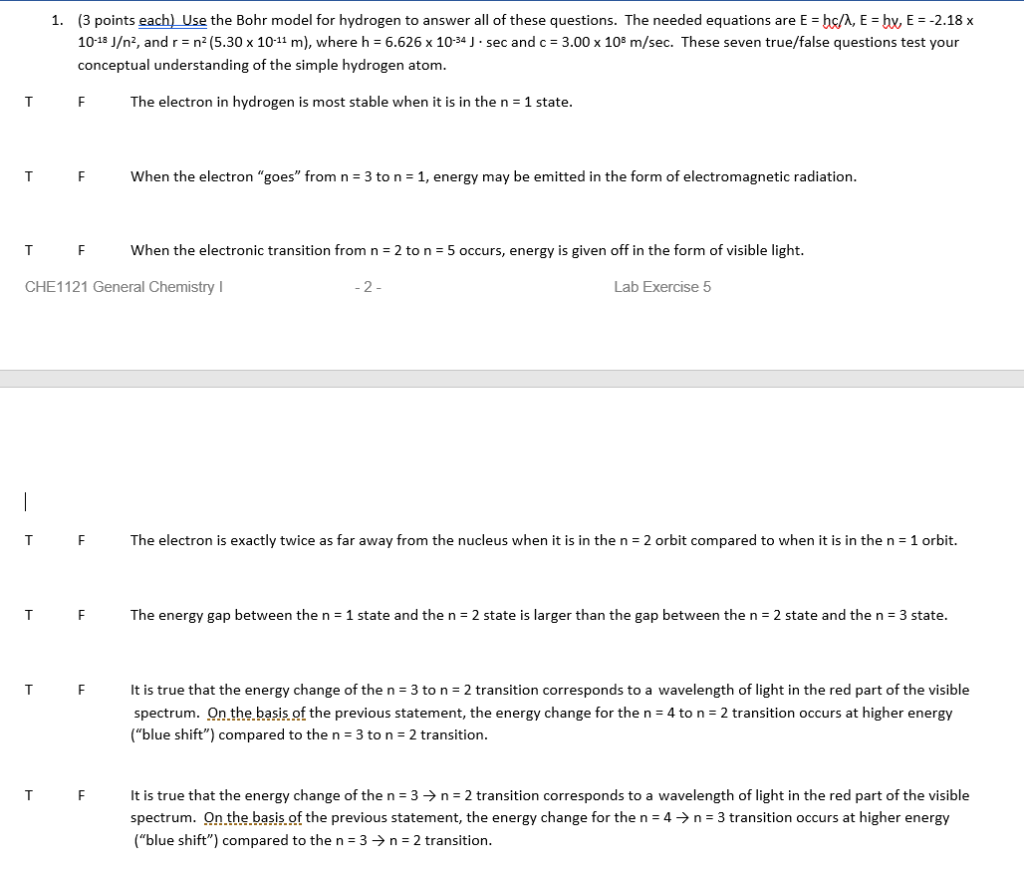
Solved 3 Points Each Use The Bohr Model For Hydrogen To Chegg Com
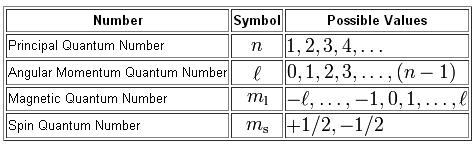
Which Of The Following Is Not A Valid Set Of Quantum Numbers A N 3 L 0 Ml 0 And Ms 1 2 B N 2 L
Molecules Free Full Text Impact Of N Alkylamino Substituents On Serotonin Receptor 5 Htr Affinity And Phosphodiesterase 10a Pde10a Inhibition Of Isoindole 1 3 Dione Derivatives Html

How Many Electrons In An Atom May Have The Following Quantum Number N 4 Ms N 3 Chemistry Structure Of Atom Meritnation Com

5 Some Digital Cordl
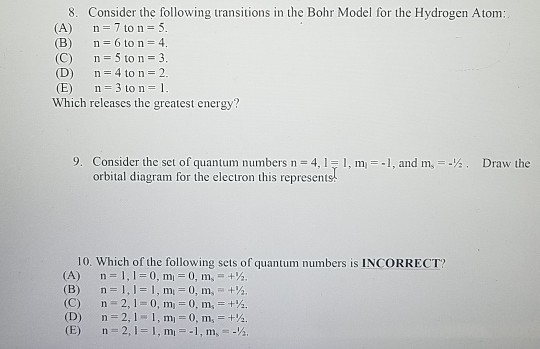
Solved I Am In Chem 1a Class And Needed To Compare My Ans Chegg Com
2

Figure 1 Generation Of U Sup Span Class Small Caps Iv Span Sup Azide Complexes
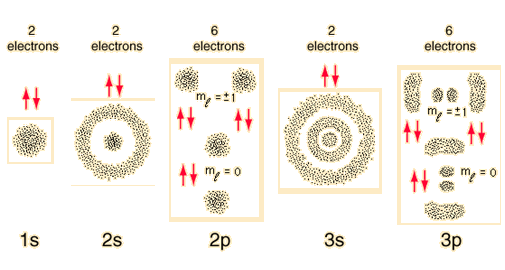
Orbitals
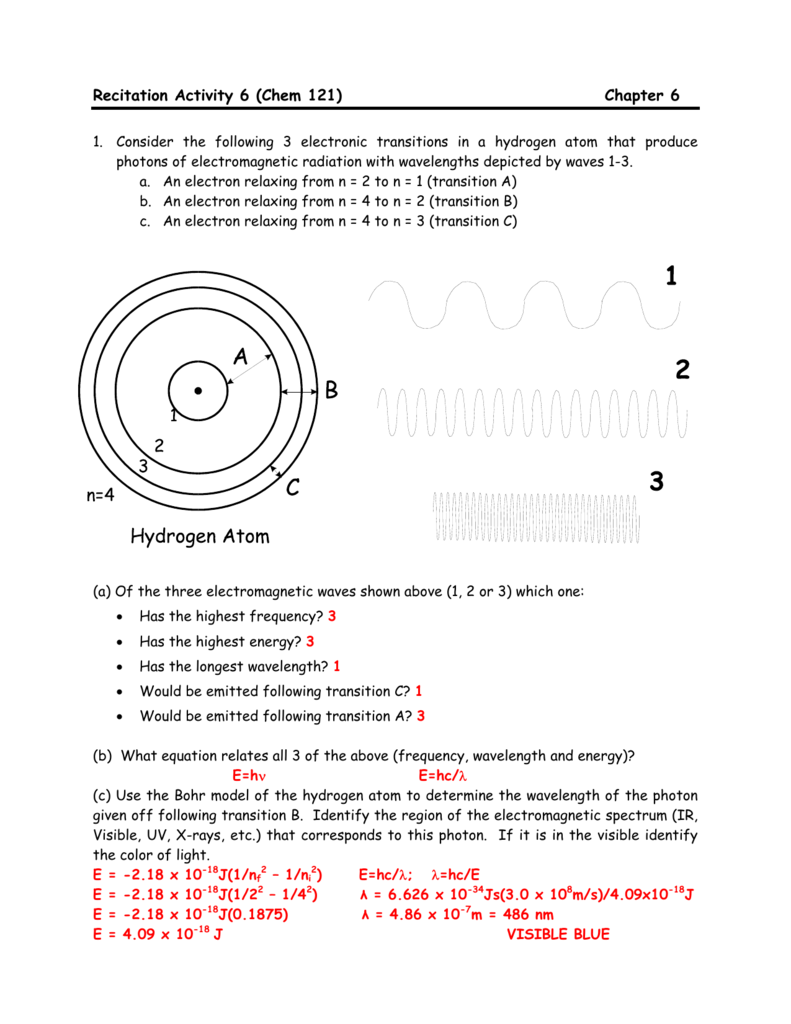
Recitation Activity 6 Chem 121 Chapter 6
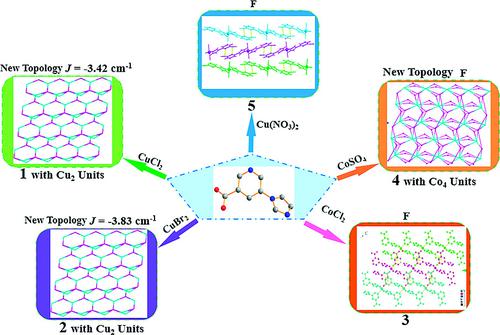
Synthesis Structures And Magnetic Properties Of Cuii And Coii Compounds Based On Asymmetric 5 1h Imidazole 1 Yl 3 Pyridine Carboxylic Acid Eur J Inorg Chem X Mol
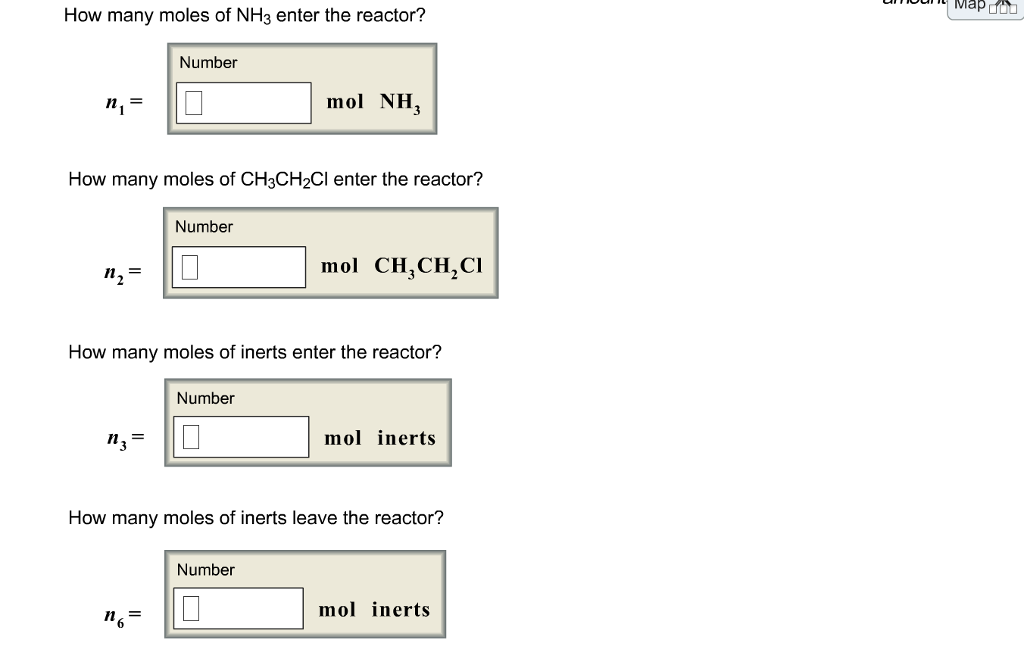
Solved Please Fine The Answer For N1 N2 N3 N4 N5 N6 N7 N8 Chegg Com
Pubs Acs Org Doi Pdfplus 10 1021 Jaa048
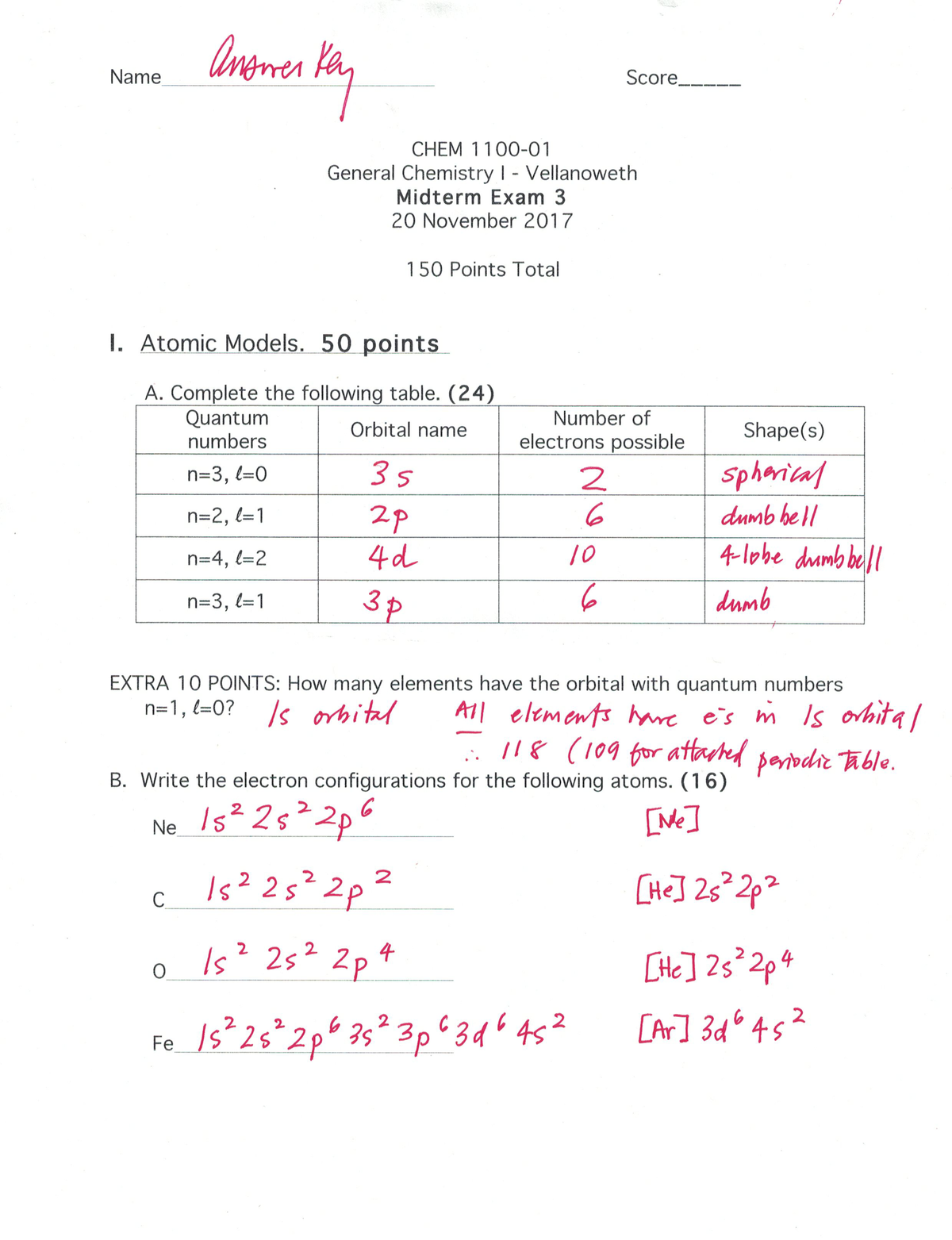
Exam Fall 17 Questions And Answers Chem 1100 Csula Studocu

Review Exam 2 Answers 09 105 Cmu Studocu

Bohr Emissions And Spectra Ppt Download
2

Chem 1031 Lecture 27 10 28 16 Atomic Spectra Bohr Model De Brolige Oneclass

General Chemistry Nitrogen Sulfuric Acid

Chemistry Section 3 Chemical Bonding Chemical Compounds Flashcards Quizlet

1 Valdosta State University

Chemistry The Central Science Chapter 6 Section 5

What Is The Aufbau Principle Chemistry Classroom Chemistry Lessons Science Chemistry

Chemistry Ia 28 Which One Of The Following Transitions Of An Electron In Hydrogen Atom Emits
Www Utc Edu Faculty Gretchen Potts Pdfs 09falltest3 Pdf

Homework 6 For Chemestry Chemistry I Che 1401 Studocu

Review Inorganic Chemistry Chemistry More Than Million Compounds Are Composed Of These 116 Elements Element Is A Substance Consists Of Identical Ppt Download



