Sf 5 Lewis Structure

How To Draw The Lewis Dot Structure For Sf5 Youtube

Molecular Geometry Ck 12 Foundation

Chapter 9 Section 2
Molecular Geometry Polarity Of Molecules And Advanced Bonding Theory
Sf6 Lewis Structure How To Draw The Lewis Structure For Sf6 Youtube
Molecular Geometry Polarity Of Molecules And Advanced Bonding Theory
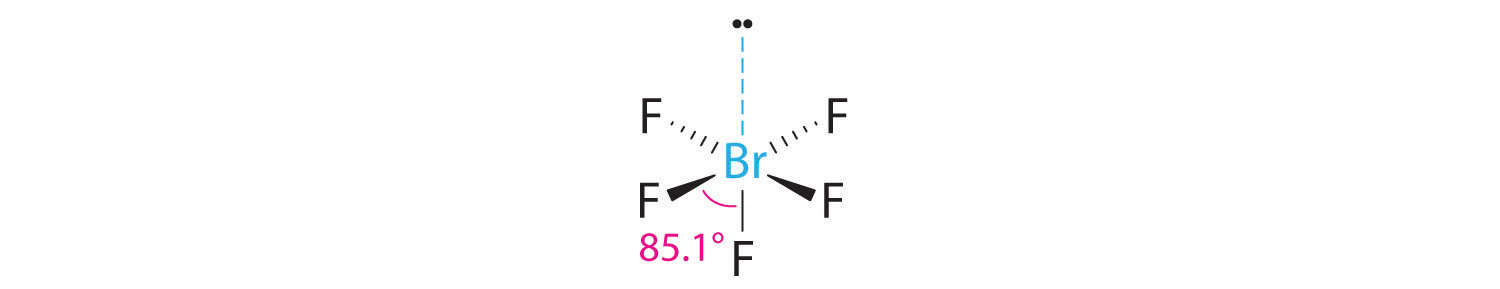
Start from the Lewis structure of the tetrafluoroborate ion, #BrF_4^(-)#.The molecule will have a total of 36 valence electrons - 7 from bromine, 7 from each of the four fluorine atoms, and one extra electron to give the ion the -1 charge.
Sf 5 lewis structure. Get your answers by asking now. For a given Lewis structure of a molecule, count the number of s and p bonds. (Opens new window.) Click the Chemical Formula to see the Lewis Structure.
A Lewis structure is a graphic representation of the electron distribution around atoms. The SF 5-ion is formed when SF 4 (g) reacts with fluoride salts containing large cations, such as CsF(s). This demo will convert a skeletal figure, provided by a drawing in the HTML5 SketcherCanvas component on the left, into a Lewis Dot Structure in the Canvas on the right.
(Read Lab K!) For a given covalent species such as PF5 or SO4 2-, you need to do the following:. Phosphorus Pentafluoride on Wikipedia. BeF 2, BCl 3, CCl 4, PBr 5, SI 6, BH 2 –, NI 3, ClF 4 +, SF 5 – Answer.
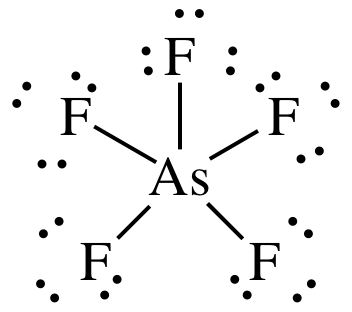
Check the post here to know about the SF6 molecular geometry and how to draw lewis structure of SF6. Sulfur tetrafluoride has 5 regions of electron density around the central sulfur atom (4 bonds and one lone pair). Arsenic (As) is the least electronegative and goes at the center of the Lewis structure.
Back to Molecular Geometries & Polarity Tutorial:. A summary of your objectives appears as Lab K. I can't figure it out!!!.
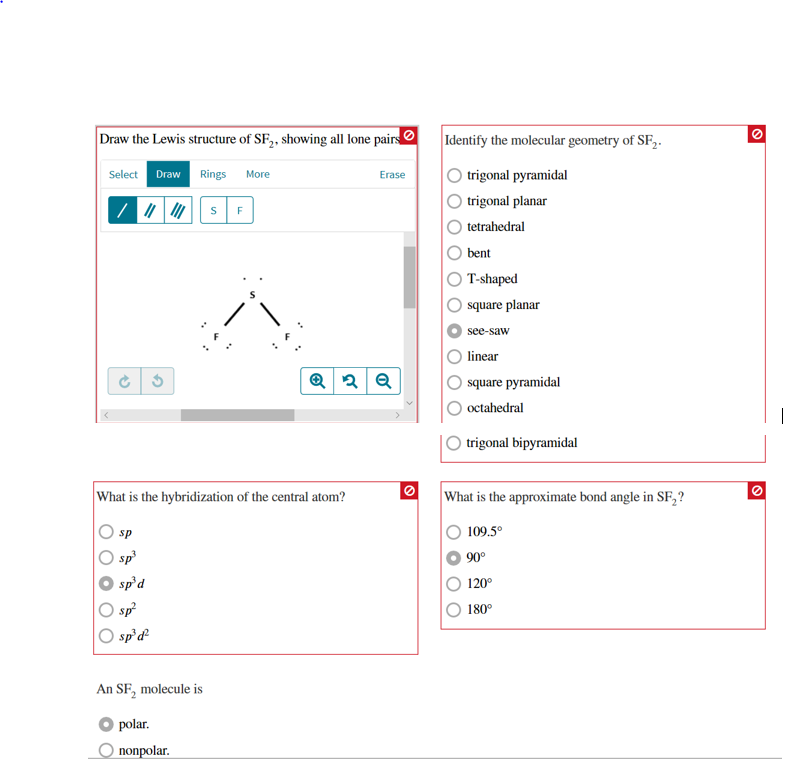
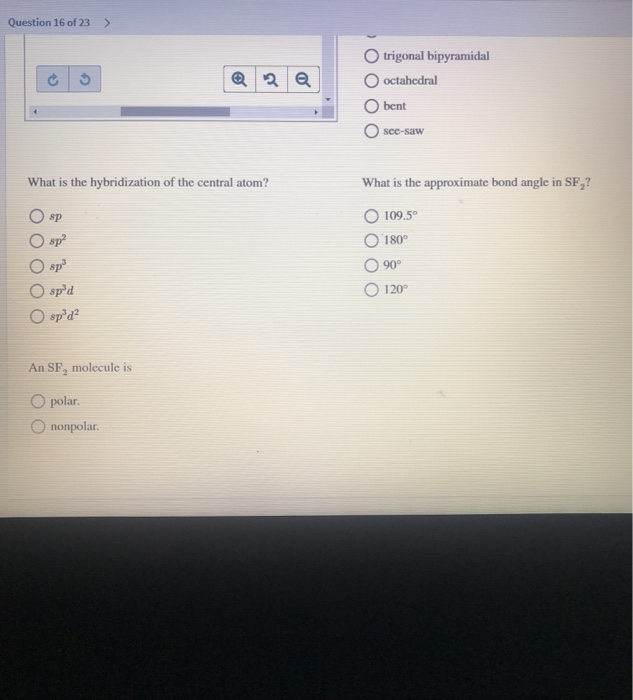
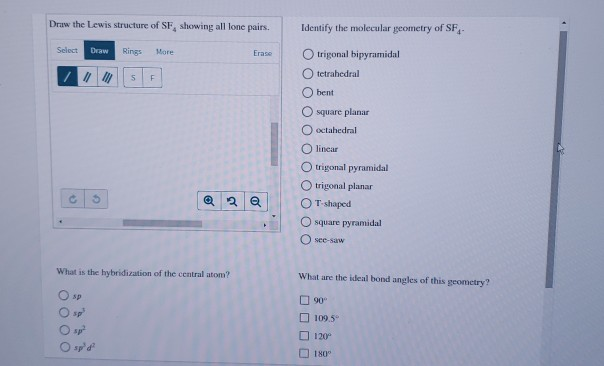
Draw the Lewis structures for SF 4 and SF 5-, and predict the molecular structure of each. 90° and 1° d. The Lewis Structure, or Lewis Dot Diagram, shows the bonding between atoms of a molecule and any electrons that may exist.
There are four covalent bonds in the skeleton structure for SF 4. Search 100+ Lewis Structures on our site. (iii) Identify the geometry of the SF 5 − anion that is consistent with the Lewis structure drawn in part (b)(i).
Expert Answer 100% (1 rating) Previous question Next question Get more help from Chegg. Sulfur Hexafluoride on Wikipeida. Sulfur in SF 4 is in the formal +4 oxidation state.Of sulfur's total of six valence electrons, two form a lone pair.The structure of SF 4 can therefore be anticipated using the principles of VSEPR theory:.
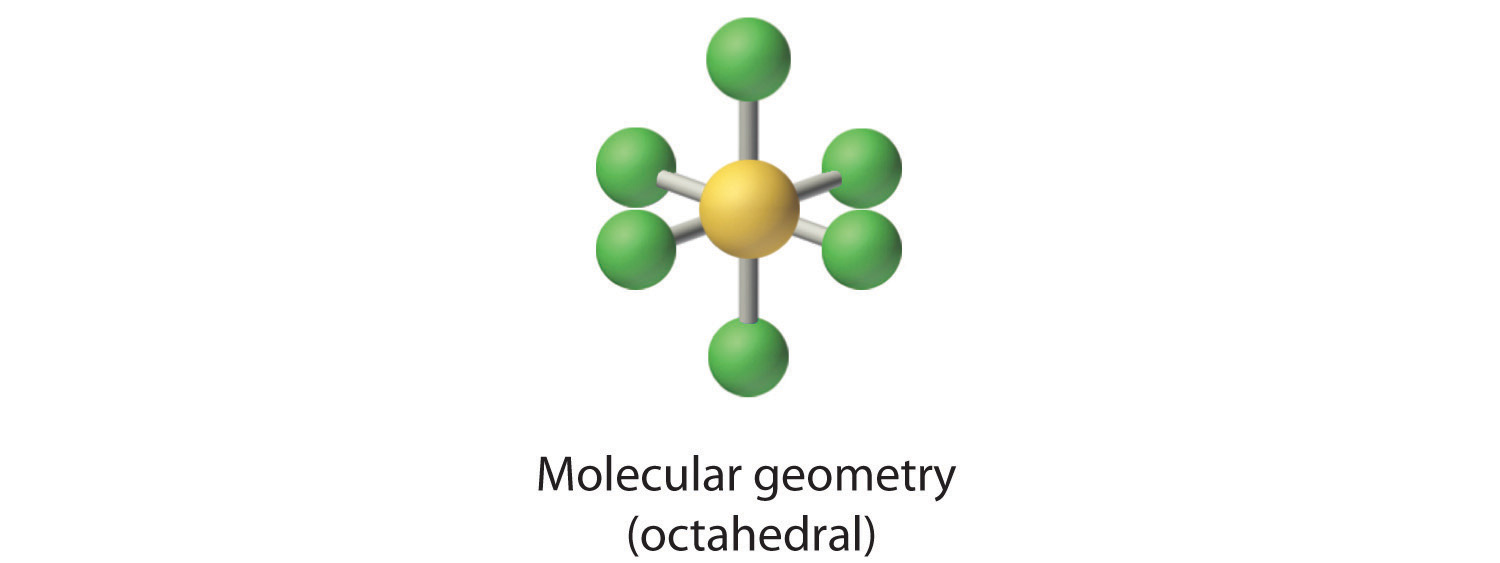
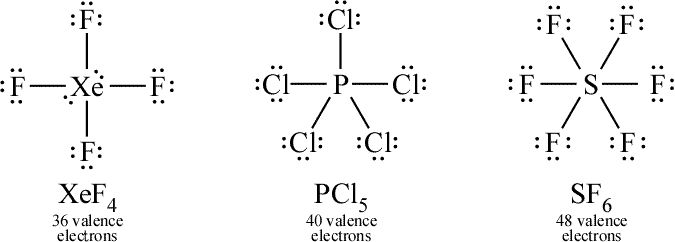
Because this requires using eight valence electrons to form the covalent bonds that hold the molecule together, there are 26 nonbonding valence electrons. Sp3d2 One point is earned for the correct hybridization. The answer is A) square planar.
Get your answers by asking now. There are five groups around sulfur, four bonding pairs and one lone pair. (ii) Identify the type of hybridization exhibited by sulfur in the SF 5 − anion.
90° and 180° e. Lewis structures, also known as Lewis dot diagrams, Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. Molecular Geometry & Polarity Tutorial.
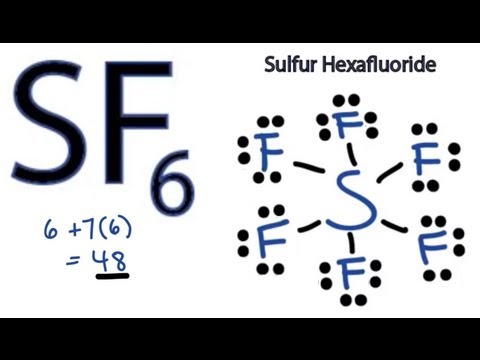
Oxygen's valency is only one. This lesson aligns with NGSS Performance Expectation:. For the SF6 Lewis structure we first count the valence electrons for the SF6 molecule using the periodic table.
90° and 1° D. Join Yahoo Answers and get 100 points today. Consider the Lewis structure for sulfur tetrafluoride (SF 4) which contains 34 valence electrons.
SO 4 2− g. Iodine is below Period Two on the periodic table so it can have an expanded octet (hold more than eight valence electrons). F 3 S—SF k.
See separate tutorial on how to write Lewis Structures. 1. Click and drag the molecle to rotate it. Drawing the Lewis Structure for SF 6.
Give the name of the electronic arrangement and the name for the molecular geometry for each of the species. Therefore this molecule is nonpolar. (b) What is the molecular geometry?.
Draw the best Lewis Dot Structures for each of the following species. U should also count bond pair to deciede the geometry ur answer lies in that. Construct and revise an explanation for the outcome of a simple.
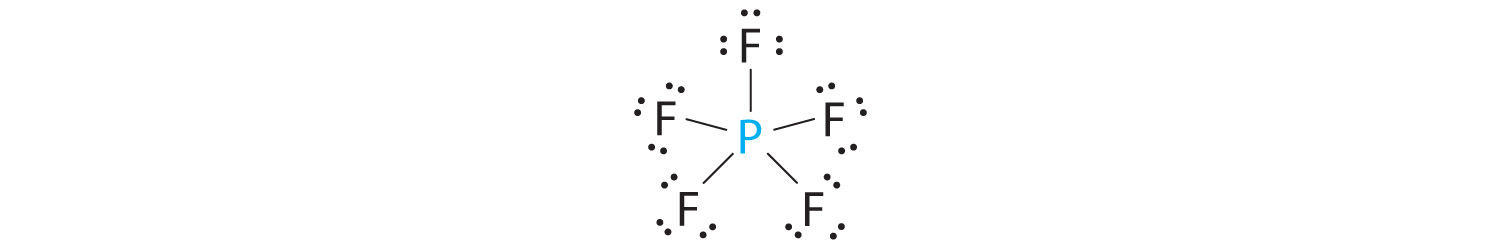
There are a total of 40 valence electrons in AsF 5. (a) What is the electron-group geometry, according to VSEPR theory?. This feature is customizable with publication quality graphics output in ChemDoodle 2D.
A step-by-step explanation of how to draw the SF5- Lewis Dot Structure. Drawing the Lewis Structure for IF 5. Get the free "Lewis structure" widget for your website, blog, Wordpress, Blogger, or iGoogle.
The bond angels in SF5+ are expected to be:. This tutorial is not meant to show you how to draw Lewis structures, but how to assign formal charges, after you have one to look at. Find more Chemistry widgets in Wolfram|Alpha.
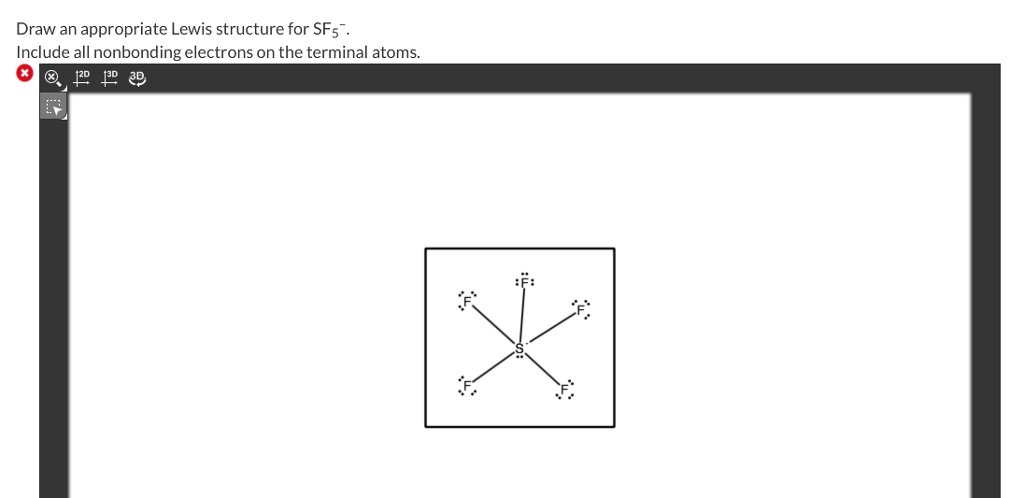
Include all nonbonding electrons on the terminal atoms. Draw the best Lewis Dot Structure for each of the following species. What are the lewis structures for:.
Back to Molecular Geometries & Polarity Tutorial:. The total number of bonds formed by sulfur with two oxygen atoms is four. So in my tutorial question, we are asked to draw the valence orbital diagram for F, S, S-, S*- and S hyb.
The molecular geometry is square pryamidal and the bond angles are 90 degrees. Draw the best Lewis structure. Ask Question + 100.
Lewis structures don't tell us everything, but along with molecule geometry and polarity they are hugely informative. The molecular geometry of SF 5 Cl is octahedral with asymmetric charge distribution on the central atom. By this method, the number of electrons is shown explicitly and the type of.
Examine each atom in the Lewis structure, one at a time. Use Lewis structures to determine which two of the following are unstable:. Among these, one is sigma bond and the second one is pi bond.
SF5- valence she'll orbital diagrams question When we draw the Lewis structure, the best structure is the one where S has the negative formal charge and each fluorine is singly bonded to the sulfur. I need an answer ASAP!. Stibine | SbH3 or H3Sb | CID 9359 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety.
Orthosilicate | SiO4(4−) or O4Si-4 | CID - structure, chemical names, physical and chemical properties, classification, patents, literature, biological. This builds on their prior knowledge of how to write Lewis Structures for Ionic Compounds from Unit 3 Lesson 4 and students knowledge of Covalent Compounds from Unit 3 Lesson 6. Molecular Geometry & Polarity Tutorial.
This is a slightly rare molecule, but here is one similar:. 90°, 1° and 180°. Therefore SF 5 Cl is polar.
Consequently, the molecule has two distinct. The bond angles in SF5+ (Draw the Lewis structure) are expected to be A. Get more help from Chegg Get 1:1 help now from expert Chemistry tutors.
It will be a square-based pyramid. The molecular geometry of PF 5 is trigonal bipyramidal with symmetric charge distribution. The Lewis structure of SF4 is the combination of 34 valence electron and 5 electron pairs around the Sulfur, in which there are four bonding pairs and one lone pair.
The electron pair distribution and the molecular geometry are different if there are lone pairs of electrons present. Here, you will find details like the shape of Sulfur Hexafluoride and whether the SF6 molecule is polar or nonpolar. The bromine atom will be bonded to each of the four fluorine atoms via single bonds for a total of 8 of the 36 valence.
Draw the most important Lewis structure for {eq}(SF_5)^- {/eq}. A) BH 2 – b) NI 3 c) ClF 4 + d) SF 5 –. The answer to “Use Lewis structures to determine which two of the following are unstable:.
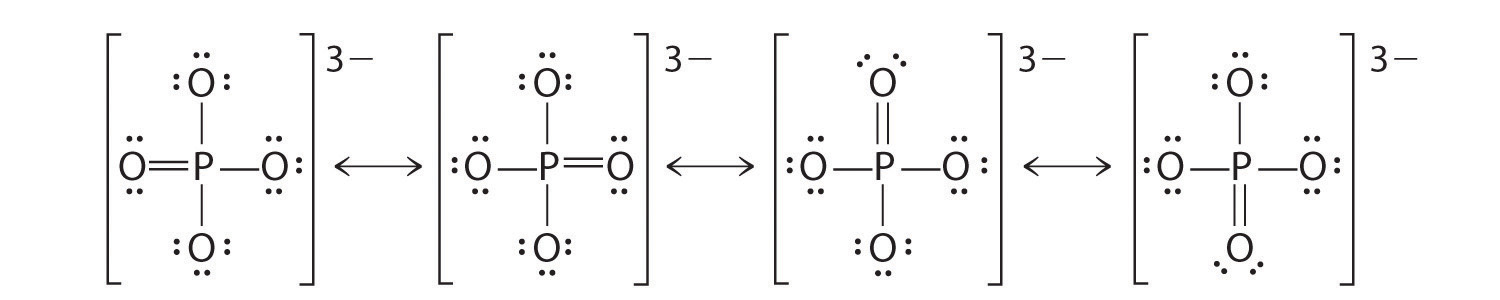
Get 1:1 help now from expert Chemistry tutors. Draw the Lewis Structure and determine its ABE formula. Hence each oxygen makes two bonds with sulfur atom.
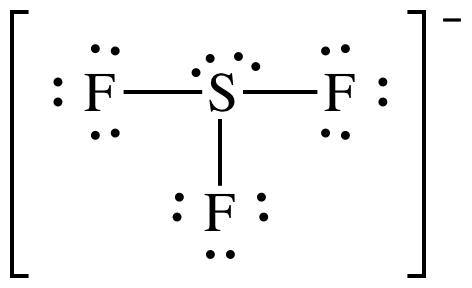
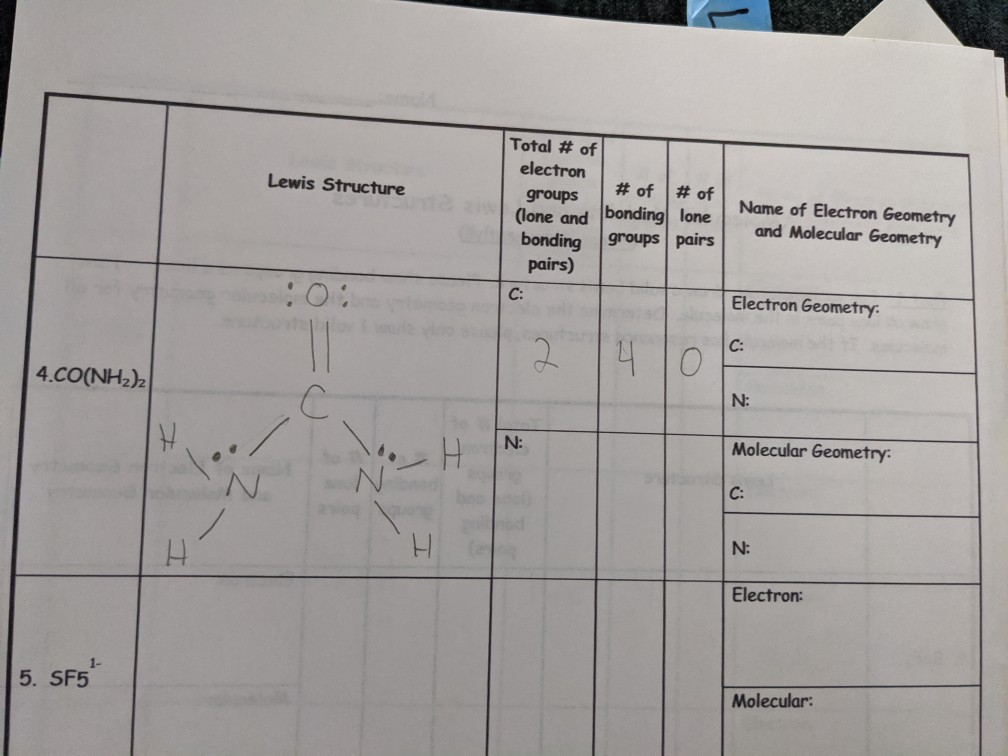
C sf 5 sulfur atom valence electrons is 6electrons c) SF 5 − Sulfur atom Valence electrons is -6 Electrons from the 5 florin atoms-5 (15) (-)charge electron-1 Thus, total valence electrons →-6+5+1=12. SO 3 2− f. Once we know how many valence electrons there are in SF6 we can distribute them around the central atom and attempt to fill the outer shells of each atom.
A) BeF 2 b) BCl 3 c) CCl 4 d) PBr 5 e) SI 6. The sulfur atom has six valence electrons and each fluorine has seven valence electrons, so the Lewis electron structure is With an expanded valence, this species is an exception to the octet rule. Sulfur's valency may be 2 or 4 or 6.
In this lesson students learn how to write Lewis Structures for covalent compounds. 90° and 180° E. Draw the best Lewis Dot Structure for each of the following species.
The reason for learning to draw Lewis structures is to predict the number and type of bonds that may be formed around an atom. The Se will be the central atom, with one lone pair and 5 Se-F bonds. The Lewis Structure for Li is Li with one dot to the right of the element.
(the structure must include lone pairs of electrons, which may be represented as dashes). Drawing the Lewis Structure for SF 6. Drawing the Lewis Structure for AsF 5.
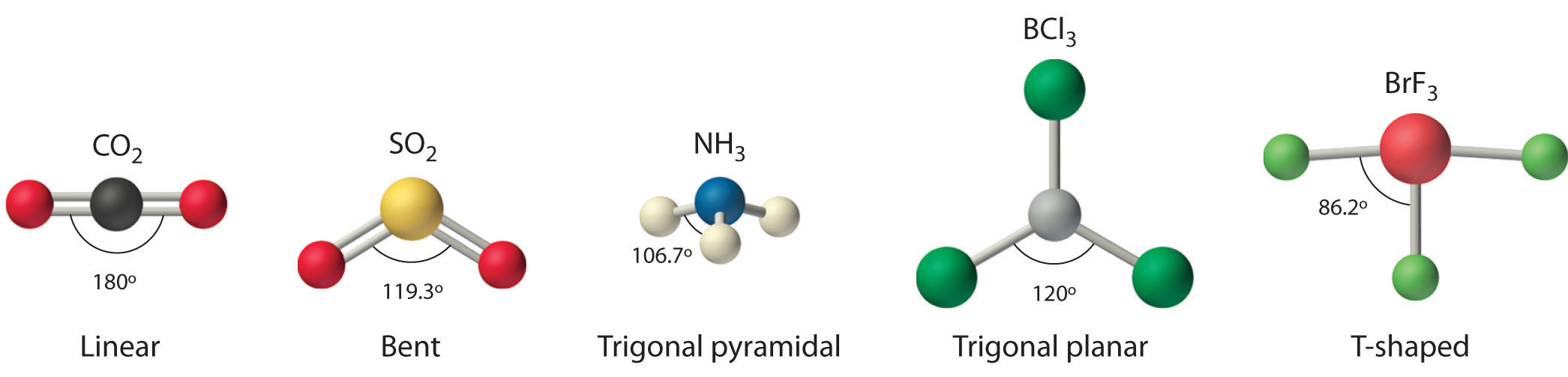
These are arranged in a trigonal bipyramidal shape with 102° F-S-F bond angles between the equatorial fluorine atoms and 173° between the axial fluorine atoms. (a) SF2 (b) SF3 (c) SF4 (d) SF5 (e) SF6 FREE Expert Solution 84% (432 ratings). (e) SF6.” is broken down into a number of easy to follow steps, and 22 words.
Write the Lewis structure. For each of the following molecules or ions that contain sulfur, write the Lewis structure(s), predict the molecular structure (including bond angles), and give the expected hybrid orbitals for sulfur. Draw an appropriate Lewis structure for SF5–.
For homework help in math, chemistry, and physics:. Having determined the arrangement of electron pairs, take off one “arm” of the structure for every lone pair present. Give the name of the electronic arrangement and the name for the molecular geometry for each of the species in question #1.
Structure, properties, spectra, suppliers and links for:. Drawing the Lewis Structure for IF 5. When you are finished drawing your 2D structure, click on the Get Lewis Dot Structure button to see the result.
It is a see-saw shape, with S at the center.One of the three equatorial positions is occupied by a nonbonding lone pair of electrons. Acetone (C 3 H 6 O) AsCl 3 (Arsenic Trichloride) AsF 3 (Arsenic Trifluoride). Five bonds, three lone pairs per F, and +1 charge on S, the central atom.
In the Lewis structure for IF5 you'll need to put a total of 12 valence electrons on the Iodine atom in order to draw the Lewis structure. We should put brackets and a negative. A Lewis structure also helps to make a prediction about the geometry of a molecule.
6 + 4(7) = 34. Also, I have mentioned some of the best usages of Sulfur Hexafluoride to help you know more about it. 90°, 1°, and 180°.
This electron arrangement is known as ‘Trigonal Bipyramidal.’. A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds. The atoms and molecules can be represented by placing the electrons in the ultimate shell as dots.
A step-by-step explanation of how to draw the SF5+ Lewis Structure. Trigonal bipyramidal, if it matters.
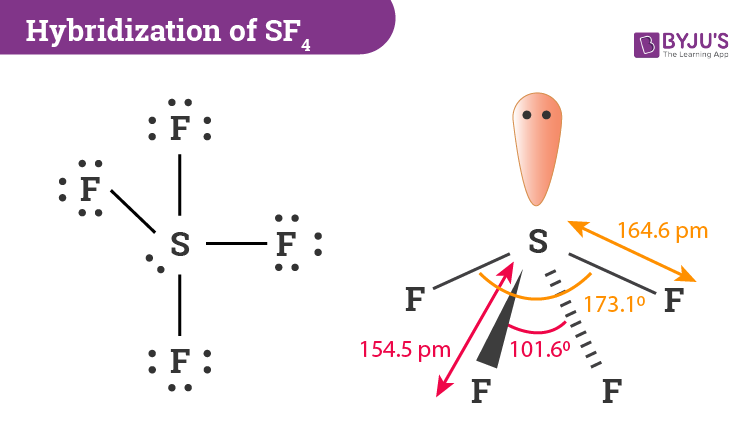
Hybridization Of Sf4 Hybridization Of S In Sulfur Tetrafluoride
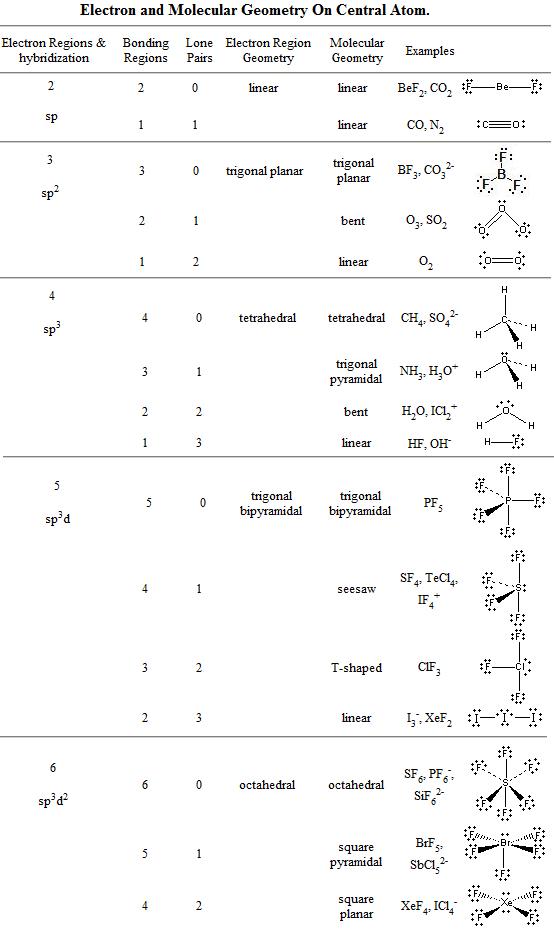
Chapter 6 3 Vsepr Molecular Geometry Chemistry Libretexts
How To Tell If A Molecule Is Polar Or Non Polar Vsepr

The Structure Of Tef 5 Isdraw A Complete Clutch Prep
How To Draw The Lewis Dot Structure For Sf5 Youtube
Chapter 8 Chemical Bonds Che 105 110 Introduction To Chemistry Textbook Libguides At Hostos Community College Library
Q Tbn 3aand9gcqgq Bgejpksjsu P0aby5jkcppyqkakzptox3sqbhscfqqlrjl Usqp Cau
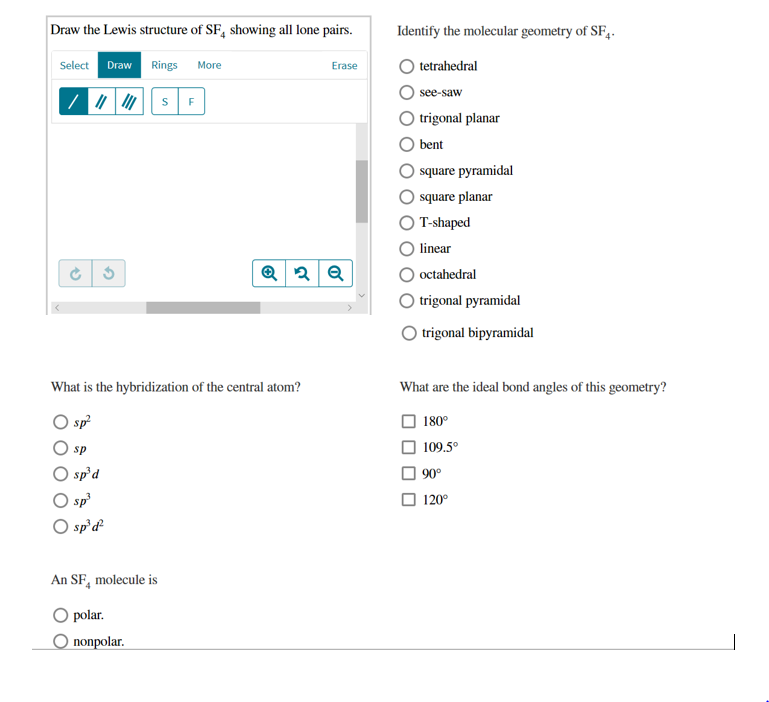
Solved Draw The Lewis Structure Of Sf Showing All Lone P Chegg Com

Formal Charges Calculating Formal Charge Youtube

Corechem Exceptions To The Octet Rule Chemprime

Asf3 Lewis Structure How To Draw The Lewis Structure For Arsenic Trifluoride Youtube

Valence Shell Electron Pair Repulsion Theory Vsepr
Www Saddleback Edu Faculty Sfier Chem 1a Files 1a Hw 2ex Pdf
Limitations With Lewis Structures Qs Study

Sf2 Lewis Structure How To Draw The Lewis Structure For Sf2 Youtube
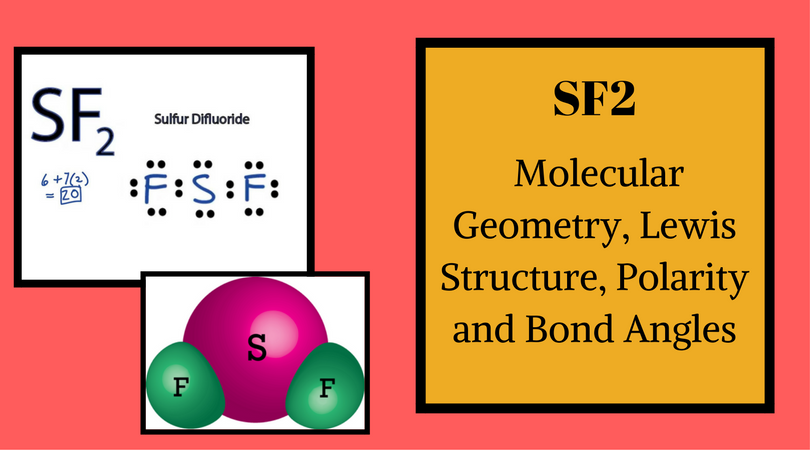
Sf2 Molecular Geometry Lewis Structure Polarity And Bond Angles
Molecular Geometry And Covalent Bonding Models
Www Sydney Edu Au Science Chemistry George 1108 Shapesofmolecules Pdf

Chapter 5 4 Exceptions To The Octet Rule Chemistry Libretexts
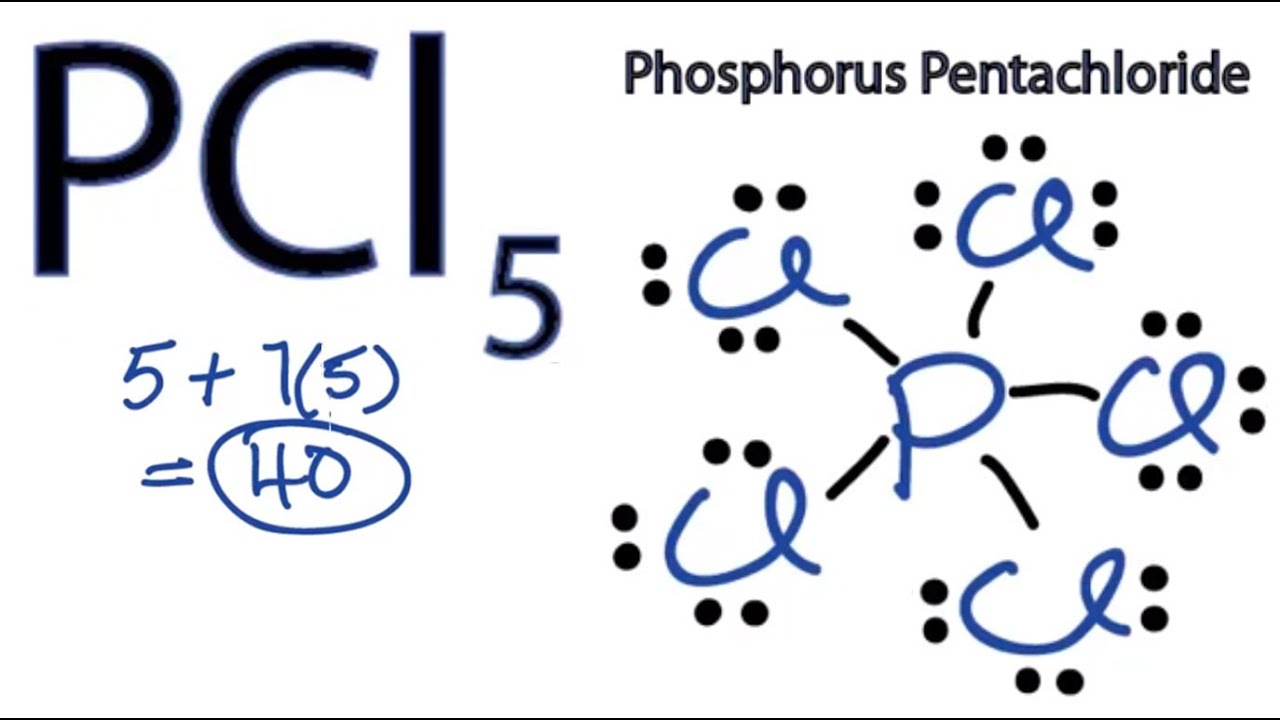
Drawing The Lewis Structure For Pcl 5
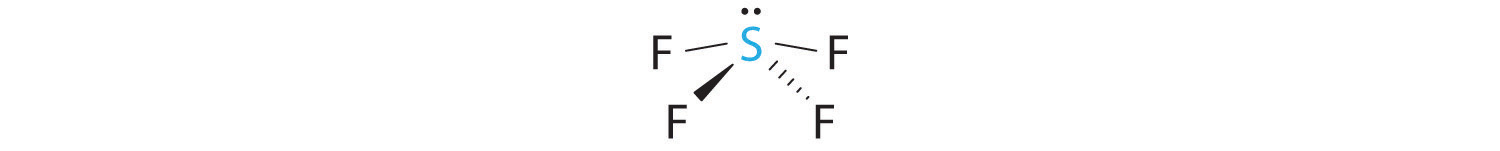
Predicting The Geometry Of Molecules And Polyatomic Ions
Lewis Electron Dot Structures Help
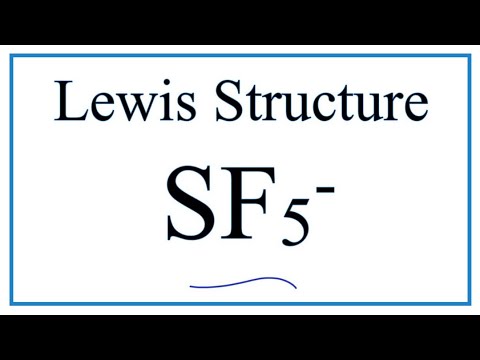
How To Draw The Lewis Dot Structure For Sf5 Youtube
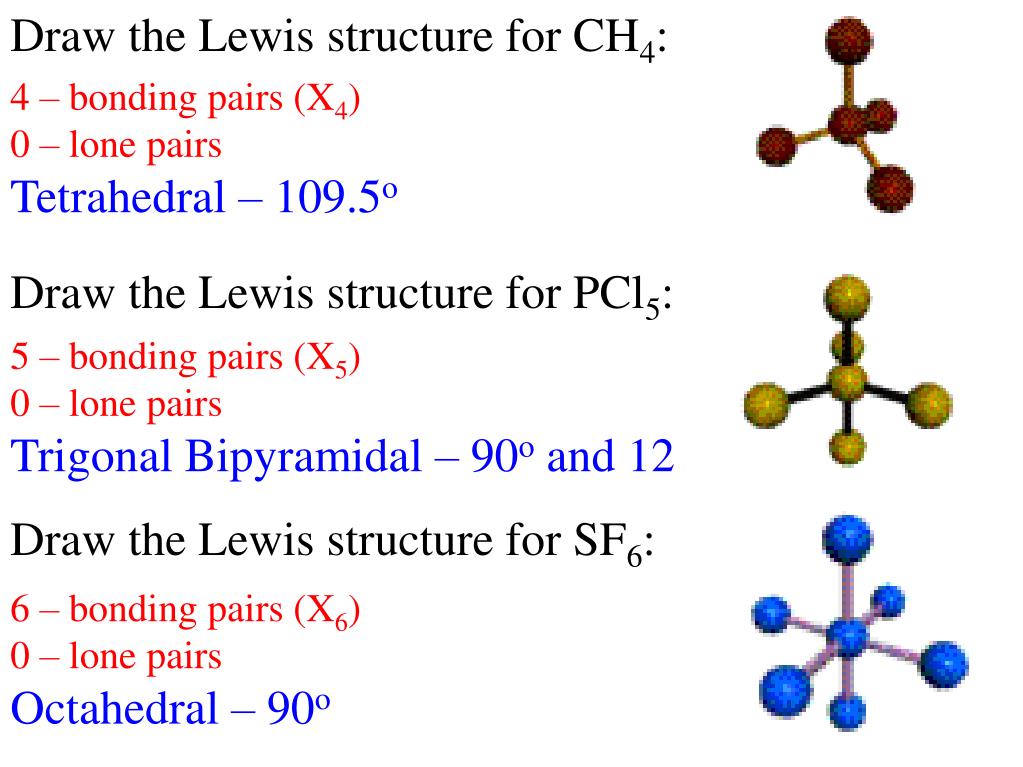
Ppt Drawing Lewis Structures And Vsepr Powerpoint Presentation Free Download Id
Secure Media Collegeboard Org Apc Ap06 Chemistry Samples Q7 Pdf
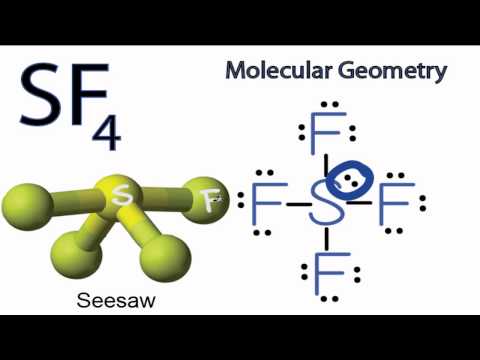
Is The Molecule Sf 4 Polar Or Non Polar Example

Solved 3 Draw The Lewis Structure Of Sf5 Chegg Com

Solved 7 Consider The Following Molecules 0 Co2 I Bf Chegg Com

Chemistry The Central Science Chapter 9 Section 2
Q Tbn 3aand9gcs Bvsjdmgidwj6eqolpvnai1byhg2cbgika0juue8pkx5e q Usqp Cau

The Bond Angels In Sf5 Are Expected To Be Clutch Prep

How Many Non Bonding Electron Pairs Are On The Central Atom Clutch Prep
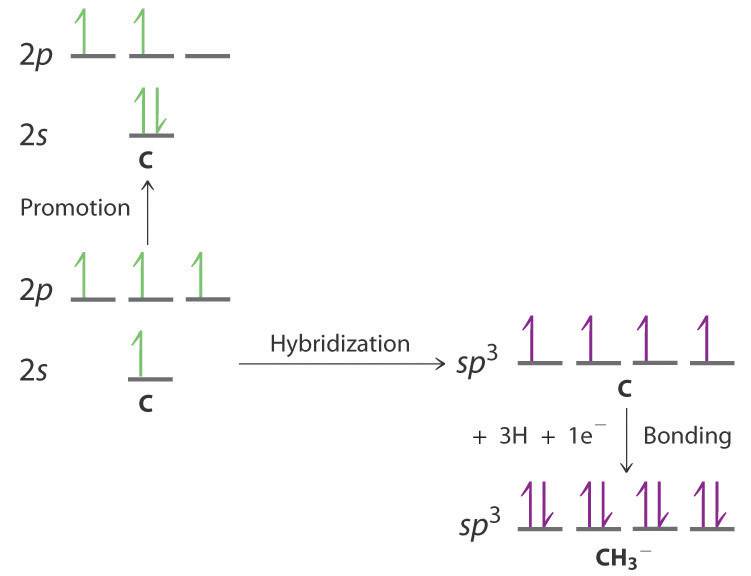
Molecular Geometry And Covalent Bonding Models
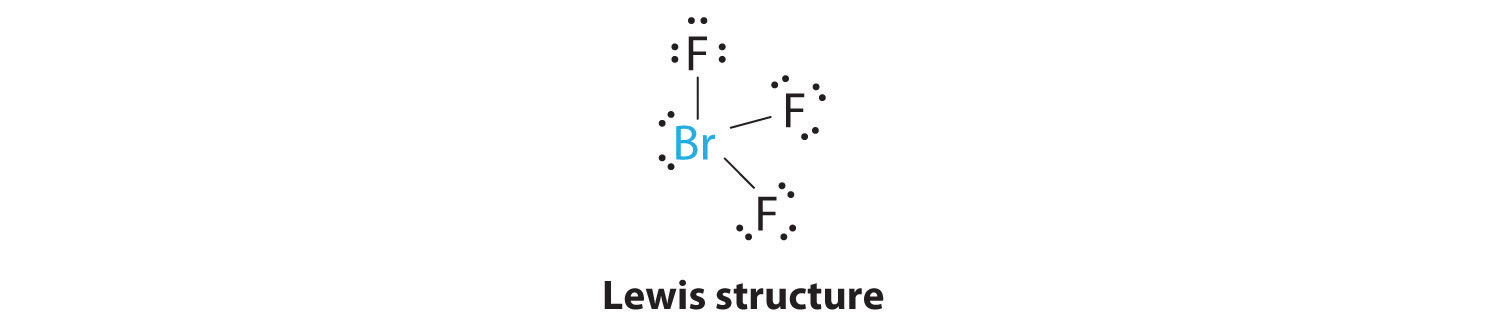
Chapter 6 3 Vsepr Molecular Geometry Chemistry Libretexts
Molecular Geometry Polarity Of Molecules And Advanced Bonding Theory

Draw The Lewis Structure For Sf 6
Chapter 5 4 Exceptions To The Octet Rule Chemistry Libretexts

Solved Determine The Best Lewis Structure For The Followi Chegg Com

Molecular Structure And Polarity Chemistry For Majors
7 3 Lewis Symbols And Structures Chemistry

Chem 121 Problem Set V Lewis Structures Vsepr And Polarity

9 7 The Shapes Of Molecules Chemistry Libretexts

How To Draw The Lewis Dot Structure For Sf5 Youtube

Sf5 Youtube

Sf4 Lewis And 3 D Structure Dr Sundin Uw Platteville
Predicting The Geometry Of Molecules And Polyatomic Ions

Vsepr Theory

Lewis Structures 5 Steps 1 Count Valence E Available If An Anion Add Charge To Valence E If A Cation Subtract Charge From Valence E 2 Draw Skeleton Ppt Download
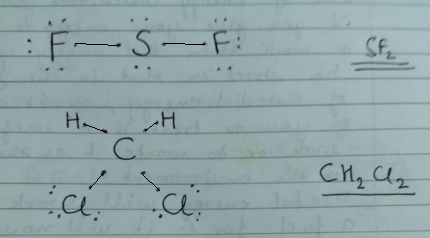
1 Write The Lewis Structure For Sf 2 Distributing The Remaining Valence Electrons So That All Three Atoms Are In Accordance With The Octet Rule 2 Construct The Lewis Structure Model For The
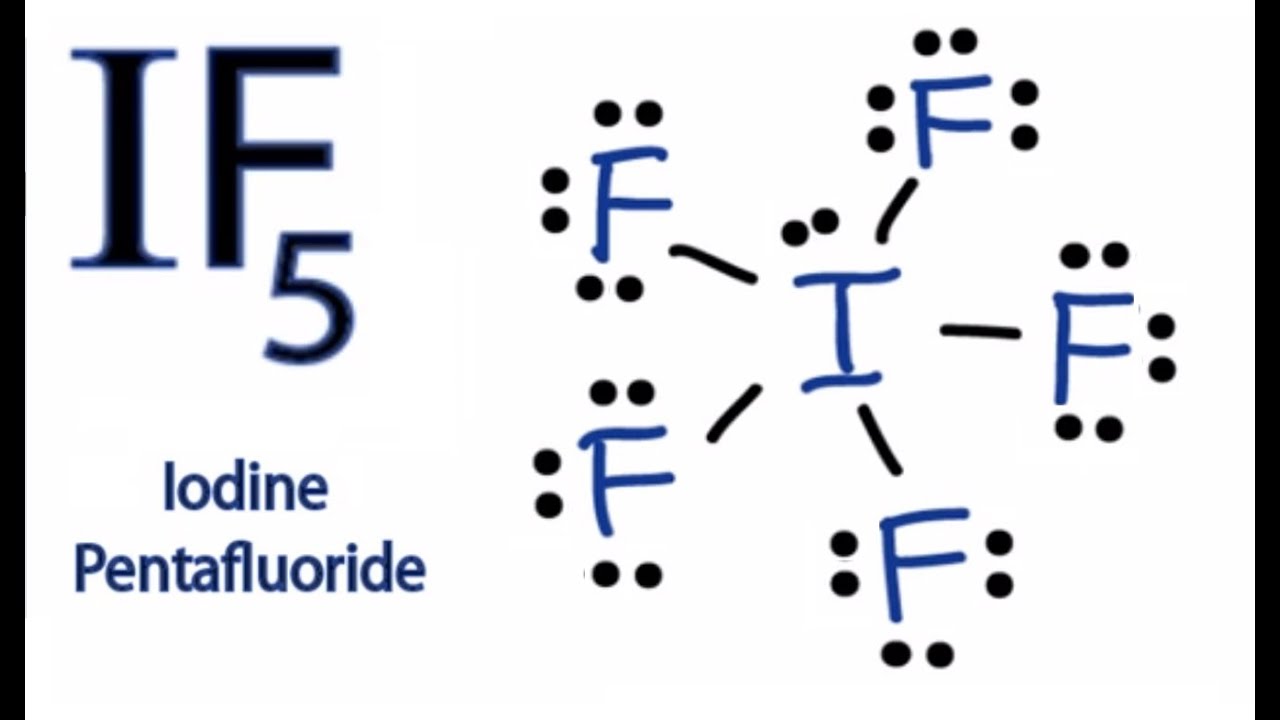
Lewis Structure For If5
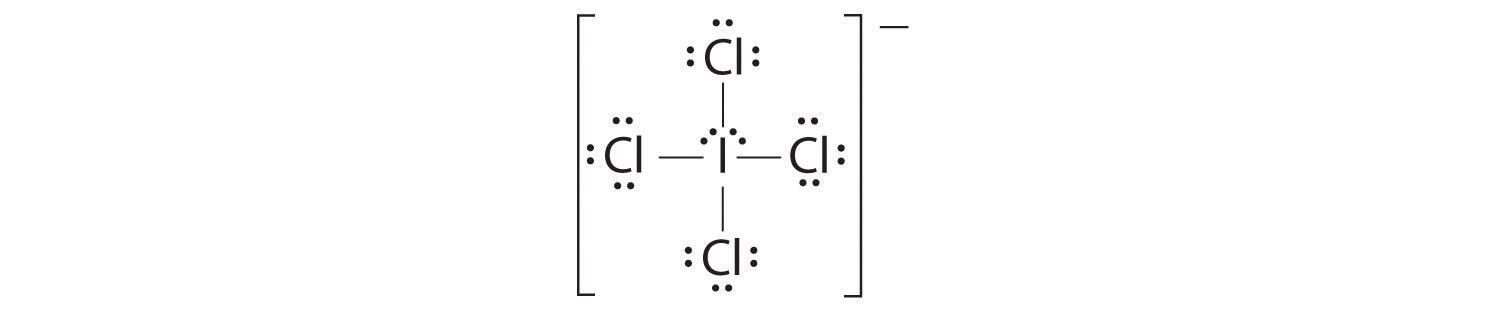
Chapter 6 3 Vsepr Molecular Geometry Chemistry Libretexts

7 3 Lewis Symbols And Structures Chemistry
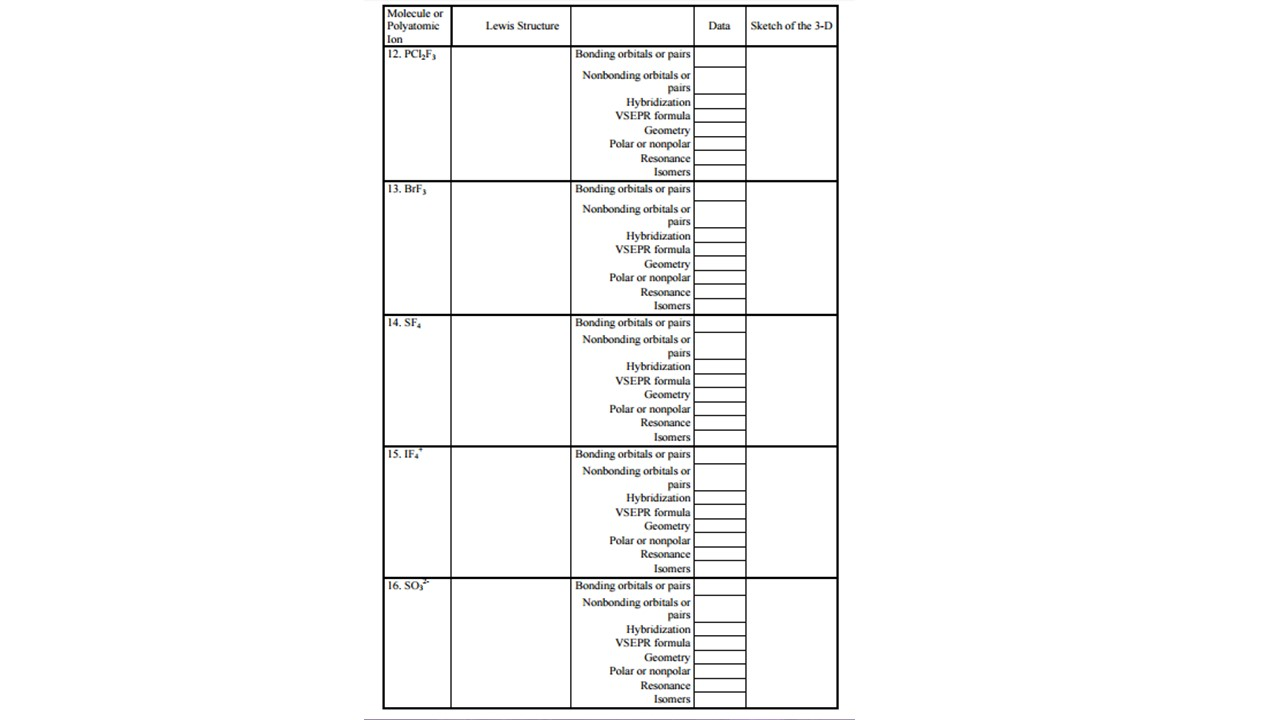
Molecule Or Polyatomic 12 Pci2fs 13 Brfs 14 Sf Chegg Com
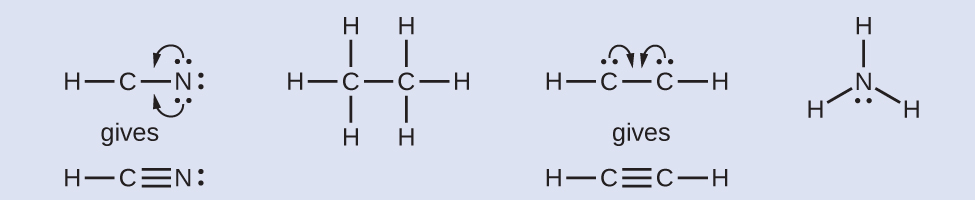
7 3 Lewis Symbols And Structures Chemistry

Answered Part V Lewis Dot Structures For Each Bartleby

Chapter 3 Molecular Shape And Structure Pages 1 11 Text Version Anyflip

5 1 Lewis Symbols And Structures General College Chemistry I
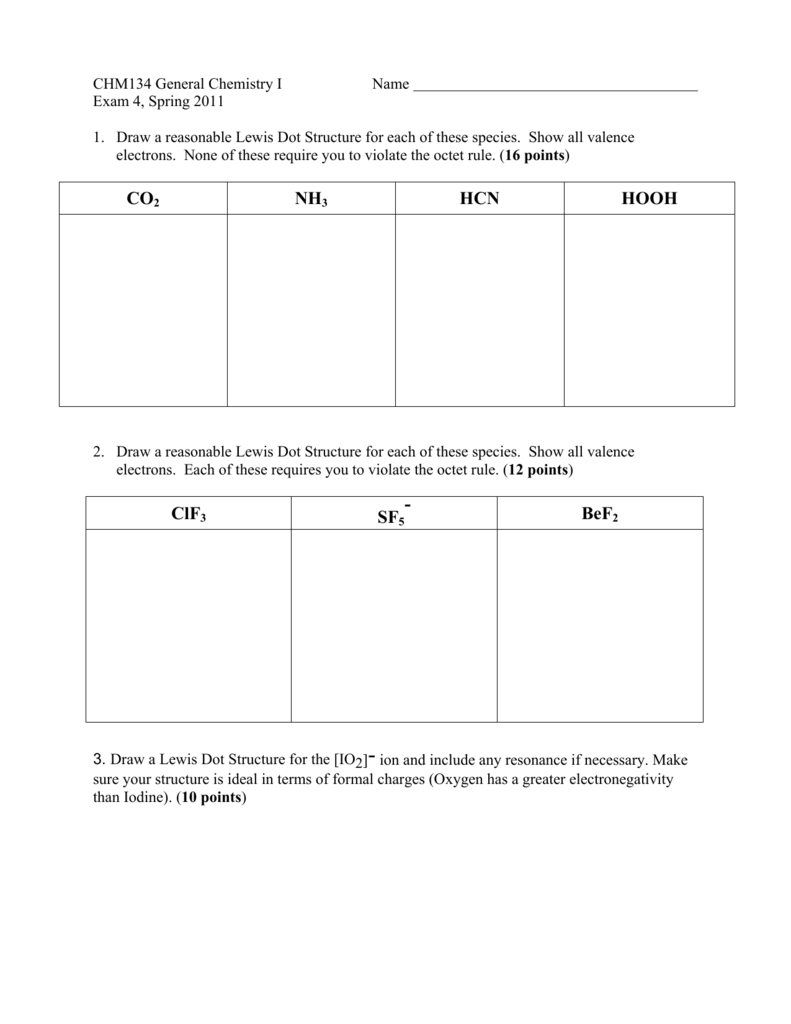
Co2 Nh3 Hcn Hooh Clf3 Sf5 Bef2
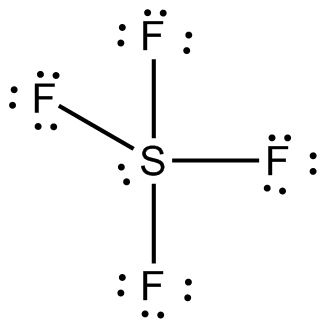
What Type Of Hybridization Does The Central Atom In Sf 4 Have Socratic

Solved 5 Points A Describe Or Draw The Lewis Structur Chegg Com
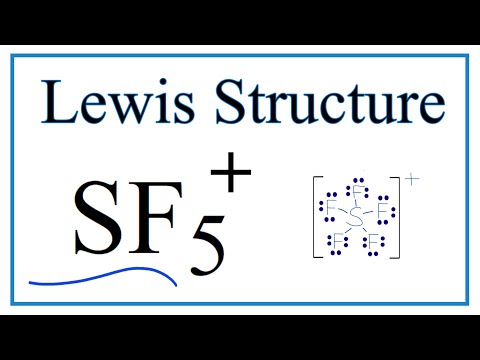
How To Draw The Lewis Dot Structure For Sf5 Youtube
Solved Draw The Lewis Structure Of Sf Showing All Lone P Chegg Com

Solved Question 3 3 Marks Draw Lewis Structures For T Chegg Com

Vsepr For 5 Electron Clouds Part 1 Video Khan Academy

The Structure Of Tef 5 Isdraw A Complete Clutch Prep
Solved Draw The Lewis Structure Of Sf Showing All Lone P Chegg Com
Solved I Think The Info Next To C Is Incorrect What Are Chegg Com
Solved Draw The Lewis Structure Of Sf Showing All Lone P Chegg Com
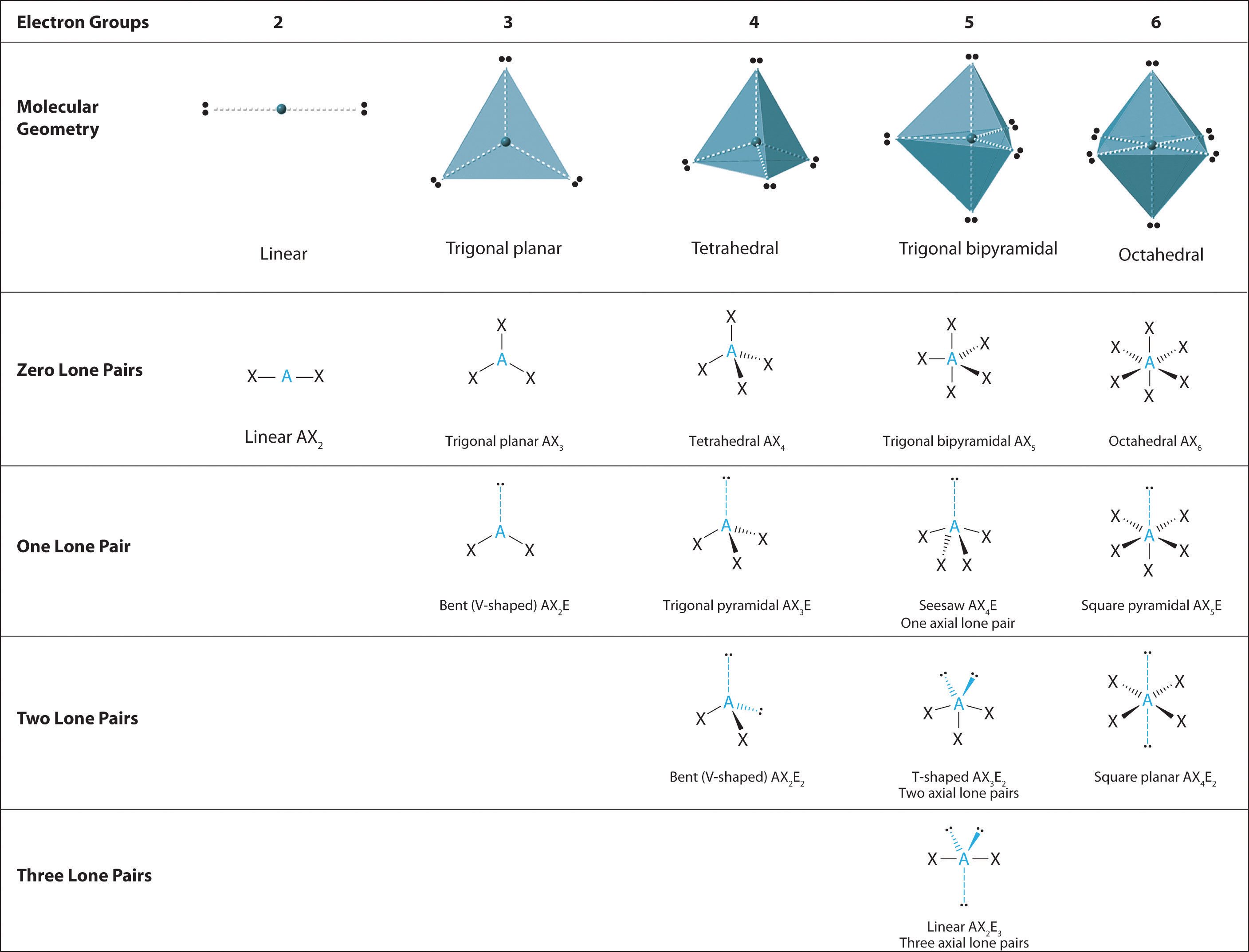
10 2 Vsepr Theory The Five Basic Shapes Chemistry Libretexts

Vsepr Theory

The Structure Of Tef 5 Isdraw A Complete Clutch Prep
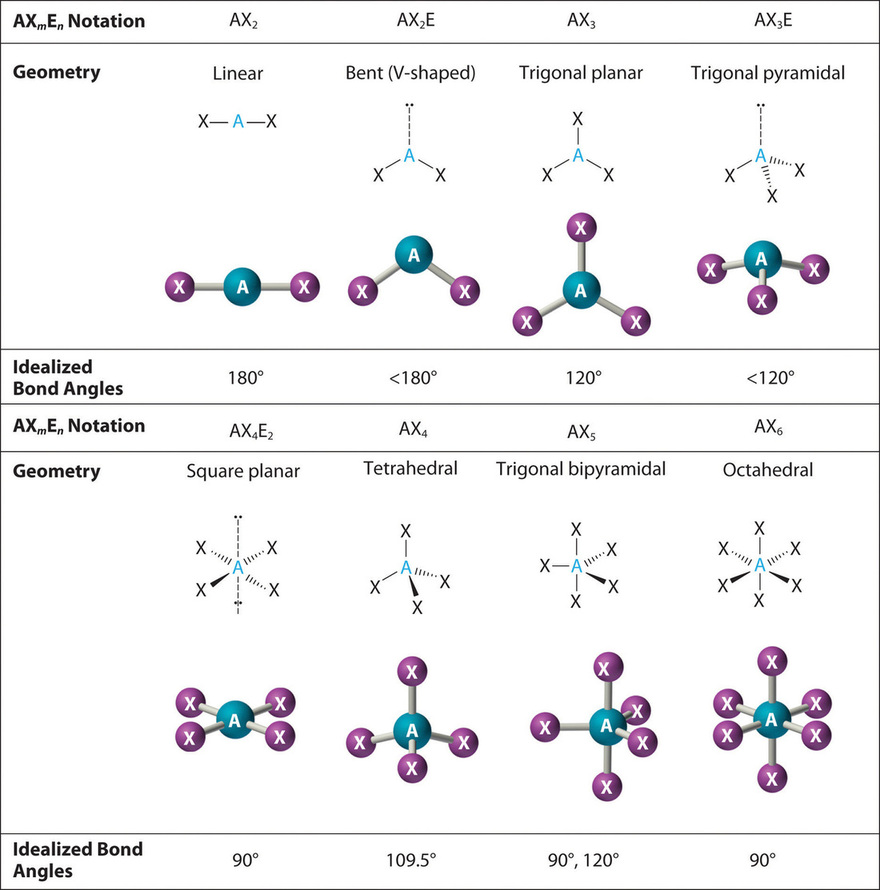
10 2 Vsepr Theory The Five Basic Shapes Chemistry Libretexts
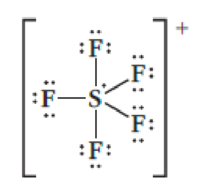
This Lewis Structure For Sf 5 Is Drawn Incorrectly What Error Was Made When Determining The Number Of Valence Electrons Bartleby
Www Sydney Edu Au Science Chemistry George 1108 Shapesofmolecules Pdf

Sulfur Chloride Pentafluoride Wikipedia

How Many Non Bonding Electron Pairs Are On The Central Atom Clutch Prep
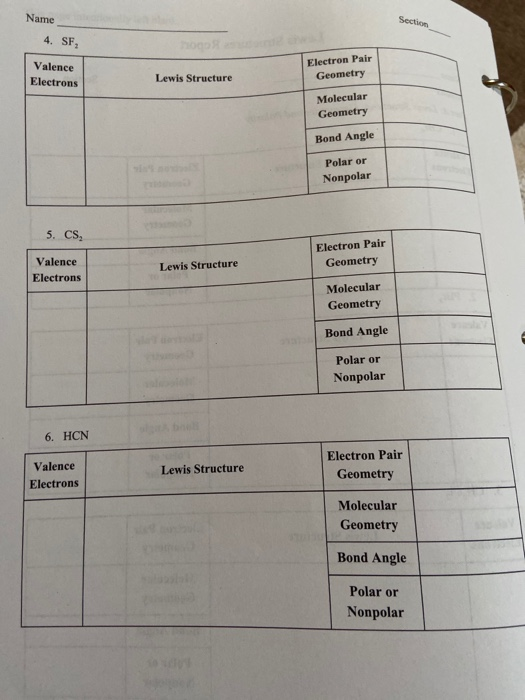
Solved Name 4 Sf Valence Electrons Electron Pair Geomet Chegg Com
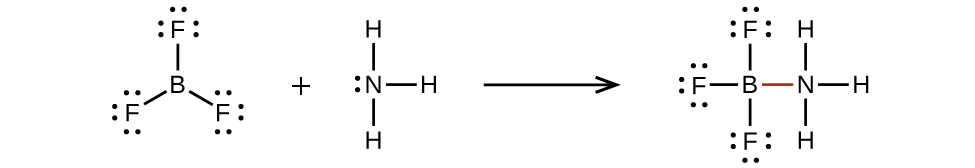
7 3 Lewis Symbols And Structures Chemistry

What Is The Molecular Shape Of Sulfur Pentafluoride Anion Chemistry Stack Exchange

Chemistry For Engineers Homework 5 Part 2 B O N D I N G Answer
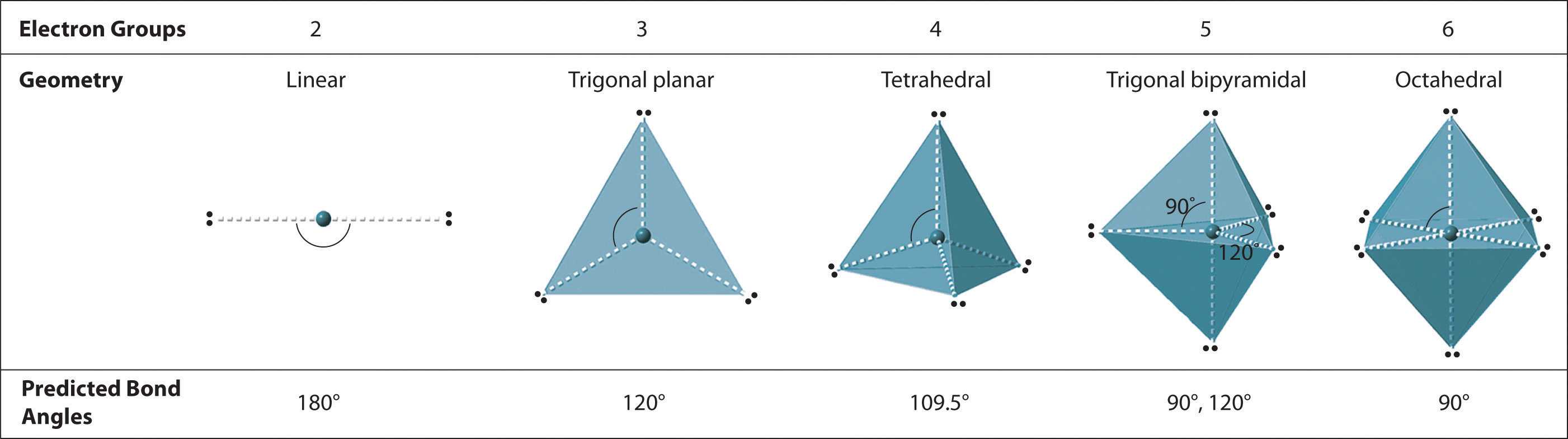
Predicting The Geometry Of Molecules And Polyatomic Ions
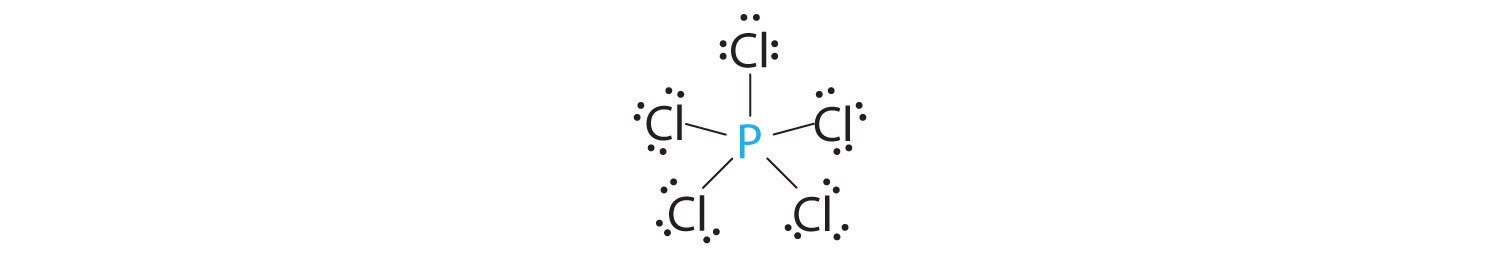
Predicting The Geometry Of Molecules And Polyatomic Ions
Secure Media Collegeboard Org Apc Ap06 Chemistry Samples Q7 Pdf
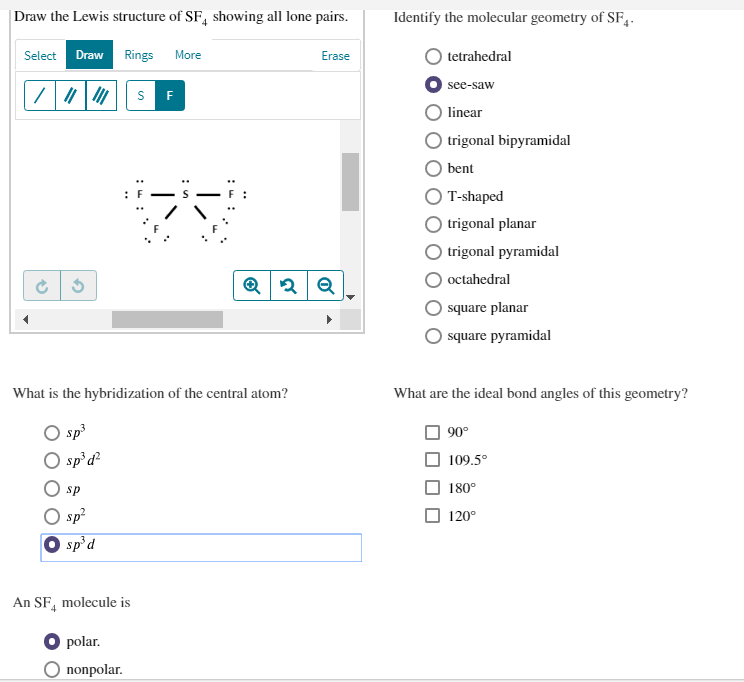
Solved Draw The Lewis Structure Of Sf Showing All Lone P Chegg Com
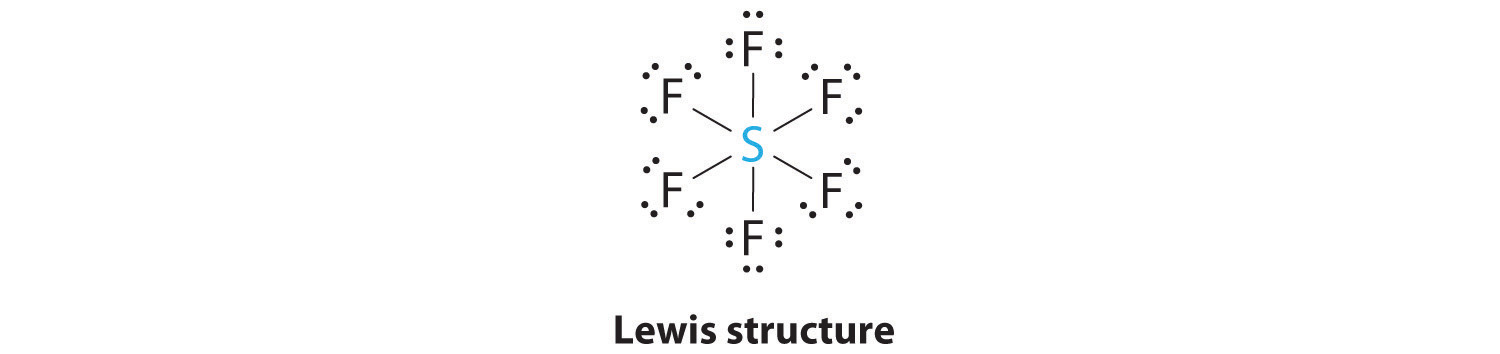
Predicting The Geometry Of Molecules And Polyatomic Ions

Best Chapter 10 Flashcards Quizlet
Solved I Know This Is The Correct Answer But I M Not Sur Chegg Com

Sulfur Tetrafluoride Wikipedia
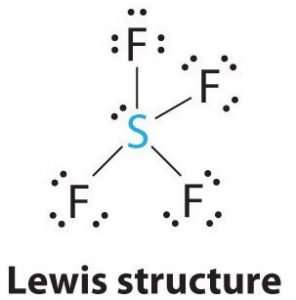
Sf4 Molecular Geometry Lewis Structure And Polarity Explained
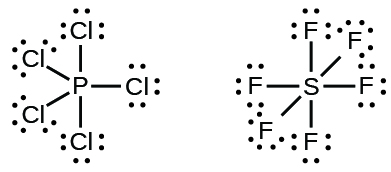
7 3 Lewis Symbols And Structures Chemistry
Q Tbn 3aand9gcrfpikcxx4sifrbdhx Jvo1pd2inisynevk0qlanonj3 Iehj60 Usqp Cau
Chapter 6 3 Vsepr Molecular Geometry Chemistry Libretexts

Sf4 Lewis Structure How To Draw The Lewis Structure For Sf4 Youtube

Vsepr Chart Valence Shell Electron Pair Repulsion Theory Sigma Aldrich
Q Tbn 3aand9gctolu6i7hvg4b1jdfjuwxn64tuo 67za9kae 0l Prpyk B1q R Usqp Cau

Sf5 Lewis Structure How To Draw The Lewis Structure For Sf5 Youtube
Chem 101 Octet Rule Violations

Lewis Symbols And Structures Introductory Chemistry



